Government
Roche 2020: Built for innovation
Roche 2020: Built for innovation

Roche is focused on finding new treatments and diagnostics that help patients live longer, better lives, and evolve the practice of medicine.
By Andrew Humphreys • andrew.humphreys@medadnews.com

F. Hoffmann-La Roche ltd.
KonzernHauptsitz
Grenzacherstrasse 124
CH-4070 Basel
Switzerland
Telephone: +41-61-688 1111
Website: roche.com
FINANCIAL PERFORMANCE
(All sales are in millions of dollars, except EPS, and were translated using the Federal Reserve Board’s average rate of exchange in 2019: SFr 0.9937)
2019
Revenue $61,856
Net income $13,583
Diluted EPS $15.72
R&D expense $11,770
1H 2020
Revenue $29,467
Net income $8,519
Diluted EPS $9.38
R&D expense $5,824
BEST-SELLING Rx PRODUCTS
(All sales are in millions of dollars and were translated using the Federal Reserve Board’s average rate of exchange in 2019: SFr 0.9937)
2019
Avastin $7,118
Rituxan/MabThera $6,518
Herceptin $6,077
Ocrevus $3,732
Perjeta $3,544
Actemra/RoActemra $2,326
Xolair $1,981
Tecentriq $1,887
Lucentis $1,838
Kadcyla $1,402
Hemlibra $1,389
Activase, TNKase $1,340
Esbriet $1,136
Alecensa $882
Pulmozyme $756
CellCept $660
Mircera $595
Gazyva/Gazyvaro $555
1H 2020
Avastin $2,853
Rituxan/MabThera $2,455
Herceptin $2,214
Ocrevus $2,089
Perjeta $1,953
Actemra/RoActemra $1,470
Tecentriq $1,305
Hemlibra $1,009
Xolair $964
Kadcyla $842
Lucentis $733
Activase, TNKase $695
Esbriet $570
Alecensa $543
Pulmozyme $354
CellCept $316
Gazyva/Gazyvaro $312
Mircera $253
Outcomes Creativity Index Score: 2
Manny Awards – N/A
Cannes Lions – N/A
LIA: Health & Wellness – N/A
Clio Health – 1
One Show: HW&P – N/A
MM&M Awards – 1
Global Awards – N/A
Creative Floor Awards – N/A
Roche has a more than 120-year history of advancing the field of medicine and bringing novel treatments and diagnostics to patients. “The patient is and will remain at the core of what we do, the reason we come to work every day,” Roche executives say. “Our autonomous research and development centers and alliances with more than 200 external partners foster a diversity of scientific approaches and agility. Our global geographical scale and reach enables us to attract talent in the leading global science clusters and to bring our diagnostics and medicines quickly to people who need them.”

“The corona pandemic continues to pose an enormous challenge worldwide. I am grateful that, in close collaboration with health authorities, we have been able to make a number of SARS-CoV-2 tests available and start several global Actemra/RoActemra phase III studies in COVID-19 pneumonia. At the same time, Roche’s regular business was significantly impacted by the pandemic in the second quarter. But we now see clear signs of recovery. Furthermore, the uptake of our recently introduced medicines and diagnostic tests continues to be strong. Based on our current assessment of the impact of the pandemic, we can confirm the outlook for the full year.” — CEO Severin Schwan
2020 Performance & Outlook
Group sales in first-half 2020 increased 1% to CHF 29.28 billion ($29.47 billion) and core EPS rose 2%, ahead of sales. IFRS net income grew 3% at constant exchange rates, due to the strong underlying core results. As a result of the continued appreciation of the Swiss franc against most currencies, the IFRS net income expressed in Swiss francs declined 5% to CHF 8.47 billion ($8.52 billion).
Sales in the Pharmaceuticals Division during the 2020 first half improved 1% to CHF 23.2 billion ($23.35 billion). Roche says the COVID-19 pandemic had an overall negative impact on the division’s sales, especially during May. Hospitalizations and out-patient visits were down, which particularly impacted sales of Ocrevus, Hemlibra, Lucentis and Rituxan/MabThera. Key growth drivers during first-half 2020 were the cancer medicine Tecentriq, the hemophilia drug Hemlibra, the multiple sclerosis product Ocrevus, Actemra/RoActemra in immunology and Perjeta in breast cancer. The new medicines (+37%) generated sales of CHF 8.9 billion ($8.96 billion) and increased by CHF 2.5 billion ($2.52 billion) at constant exchange rates over same-time 2019, more than offsetting the impact of the competition from biosimilars (CHF 2.1 billion at constant exchange rates).
U.S. sales fell 4% compared to the 2019 first half. While sales of Hemlibra, Ocrevus, Tecentriq and Actemra/RoActemra rose, competition from biosimilars for Herceptin, Avastin and Rituxan/MabThera impacted this growth as expected. Hemlibra sales advanced 80%, resulting from the ongoing U.S. rollouts. Sales for Ocrevus went up 19% and were driven by both new and returning patient demand. First-half 2020 sales of both Hemlibra and Ocrevus were partly impacted by COVID-19 effects. Tecentriq sales rose 52%, driven by growth in the new indications ES-SCLC and triple-negative breast cancer. In the United States and other countries, increased use of Actemra/RoActemra in patients with severe COVID-19 pneumonia can be observed as countries included it in their treatment guidelines, Roche says. Actemra/RoActemra is not currently approved for this use; Roche is conducting several phase III clinical trials in severe COVID-19 pneumonia.
Sales in Europe during the first six months of 2020 increased (+5%) as the strong demand for Tecentriq, Ocrevus, Hemlibra, Kadcyla, Perjeta and Actemra/RoActemra was able to offset the impact of lower sales of Herceptin (-33%) and Rituxan/MabThera (-34%). The first biosimilar versions of Avastin could come to market in Europe during second-half 2020.
In the International region (+11%), first-half 2020 growth was mostly driven by Russia and China. Growth in China resulted from a strong uptake of Perjeta and Alecensa, which was partially offset by the National Reimbursement Drug List price cut and COVID-19 impact for Herceptin, Rituxan/MabThera and Avastin.
Sales decreased in Japan 2% versus the January-June 2019 period, resulting from considerable competition from biosimilars, generics and government price cuts. This decrease was partially compensated by recently launched medicines including Tecentriq, Hemlibra and Perjeta.
Diagnostics Division sales for the January-June 2020 period grew 3% to CHF 6.08 billion ($6.12 billion). The Molecular Diagnostics business area (+61%) was the main growth contributor. Sales of the recently developed cobas SARS-CoV-2 PCR tests could offset the negative impact of the COVID-19 pandemic on products for routine diagnosis, management says. First-half growth was reported in North America (+13%), EMEA (+5%), Latin America (+6%) and Japan (+1%). In the Asia-Pacific region (-9%), decreased sales were strongly impacted by the COVID-19 pandemic shutdown in China. Overall, demand was impacted by COVID-19 in all regions in Q2 2020. Routine testing declined significantly due to a decrease in regular health checks while emergency and SARS-Co-V-2 testing rose significantly.
The core operating profit increased 2% in the Pharmaceuticals Division and 9% in the Diagnostics Division for the first six months of 2020.
Based on the current assessment of the COVID-19 impact, full-year 2020 sales are expected to grow in the low- to mid- single digit range, at constant exchange rates. Core earnings per share are targeted to increase broadly in line with sales, at constant exchange rates. Roche expects to increase the company’s dividend in Swiss francs further.
COVID-19 Pandemic Response
Ever since the early phase of the COVID-19 pandemic, we have been partnering with healthcare providers, laboratories, authorities and organizations to provide patients with the tests, treatments and care they need,” Roche management says. “The portfolio of our recently developed SARS-Co-V-2 tests as well as our existing diagnostics menu for critical care have become a significant factor in supporting patient management during the COVID-19 pandemic. Roche is working closely with healthcare providers around the world, and has significantly increased production to provide tests globally.”
According to Roche, no major manufacturing supply chain issues have been identified and the Group’s planned drug launches, filings, pivotal phase III study readouts and pivotal trial starts are largely on track.
The global phase III randomized, double-blind, placebo-controlled clinical study COVACTA was initiated to assess the safety and efficacy of intravenous Actemra/RoActemra plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia compared to placebo plus standard of care. The first patients were enrolled during early April.
On July 29, Roche reported that the COVACTA study did not meet the primary endpoint of improved clinical status in patients with COVID-19 associated pneumonia, or the key secondary endpoint of reduced patient mortality. Roche remains dedicated to continuing the Actemra/RoActemra clinical study program in COVID-19 to further explore the product in other treatment settings, including in combination with an antiviral.
The global phase III, randomized, double-blind, multicenter study REMDACTA was initiated to assess the safety and efficacy of Actemra/RoActemra plus the antiviral remdesivir, versus placebo plus remdesivir in hospitalized patients with severe COVID-19 pneumonia. In collaboration with Gilead Sciences, the clinical trial began enrollment during June. Data from the REMDACTA study are designed to supplement the phase III COVACTA trial; results were anticipated during 2020.
Results from Roche’s phase III EMPACTA study reported in September demonstrated that Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalized patients with COVID-19 associated pneumonia. EMPACTA represents the first worldwide phase III study to show efficacy with Actemra/RoActemra in COVID-19 associated pneumonia and the first trial with a focus on enrolling largely underserved and minority patients. Started in the United States during May 2020, the trial was expanded to sites in other countries, including Brazil, Kenya, Mexico, South Africa and Peru.
MARIPOSA – a worldwide phase III randomized, double-blind, placebo-controlled study – was initiated to test the safety and efficacy of 8 mg/kg vs 4 mg/kg intravenous Actemra/RoActemra plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia. Results were expected to be reported during 2020.
Roche additionally launched an internal early research program focused on the discovery of medicines for COVID-19 and is assessing a large number of potential collaborations. As of July, six Roche medicines – including Actemra/RoActemra, Esbriet, Avastin and Pulmozyme – already approved for other diseases, were being evaluated in 28 Roche or Roche-supported clinical studies in COVID-19 infection. Several new compounds also are being evaluated in pre-clinical research.
Roche and Regeneron joined forces during August in the fight against COVID-19. The companies agreed to develop, manufacture and distribute REGN-COV2, Regeneron’s investigational antiviral antibody combination, to people worldwide. REGN-COV2 could provide a much-needed treatment option for people already experiencing COVID-19 symptoms and has the potential to prevent infection in people exposed to the virus, thus slowing the spread of the global pandemic. The collaboration is anticipated to increase supply of REGN-COV2 to at least three-and-a-half times the current capacity, with the potential for additional expansion.
Roche’s Elecsys IL-6 test received FDA Emergency Use Authorization in June to help in identifying patients at high risk of severe inflammatory response in patients with confirmed COVID-19. Interleukin 6 is an early indicator for acute inflammation to aid in the management of critically ill patients, according to Roche. The test is additionally available in markets accepting the CE Mark.
Roche filed for Emergency Use Authorization (EUA) from the U.S. FDA for the Elecsys Anti-SARS-CoV-2 S antibody test, which launched in September for markets accepting the CE Mark. Roche says using the Elecsys Anti-SARS-COV-2 S antibody test, together with the Elecsys Anti-SARS-CoV-2 test that was issued EUA during May, can help to more effectively determine the percentage of a population who already have antibodies against SARS-COV-2. According to Roche, the high specificity of the Elecsys Anti-SARS-CoV-2 antibody test is crucial to determine reliably if a person has been exposed to the virus and if the patient has developed antibodies.
Pharma Product Approvals & Pipeline Updates During 2020
Regulatory authorities worldwide granted approvals for new Roche medicines, line extensions of existing products and new tests during 2020. In the second quarter alone, Roche completed phase III trial enrollment for pivotal studies in Alzheimer’s and Huntington’s disease and started four significant phase III trials in oncology.
The U.S. FDA approved Tecentriq (atezolizumab) in combination with Avastin (bevacizumab) in early June for treating people with unresectable or metastatic hepatocellular carcinoma (HCC) who have not received prior systemic therapy. This is the first cancer immunotherapy regimen approved for the treatment of unresectable or metastatic HCC. The marketing application was reviewed under the FDA’s Real-Time Oncology Review pilot and Project Orbis initiative, helping to bring the new treatment option rapidly to patients in the United States and around the globe.
During September, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended the approval of Tecentriq in combination with Avastin for the treatment of adults with advanced or unresectable HCC who have not received prior systemic therapy.
Tecentriq won U.S. regulatory clearance in May as a first-line treatment for adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression (PD-L1 stained ≥ 50% of tumor cells [TC ≥ 50%] or PD-L1 stained tumor-infiltrating covering ≥ 10% of the tumor area [IC ≥ 10]), as determined by an FDA-approved test), with no EGFR or ALK genomic tumor aberrations.
Near the end of July, the U.S. regulatory agency granted approvals for Tecentriq plus Cotellic (cobimetinib) and Zelboraf (vemurafenib) for people with advanced melanoma. The supplemental Biologics License Application for Tecentriq was approved under priority review. The review was additionally performed under Project Orbis, an initiative of the FDA Oncology Center of Excellence that provides a framework for concurrent submission and review of oncology products among international partners.
During September, Roche presented new data from multiple phase III trials of Tecentriq in triple-negative breast cancer at ESMO Virtual Congress 2020. Data from the phase III IMpassion031 trial showed that Tecentriq in combination with chemotherapy improved pathological complete response for patients with early triple-negative breast cancer (TNBC), when compared to placebo plus chemotherapy. Final overall survival data from the phase III IMpassion130 trial were consistent with previous interim analyses in patients with metastatic TNBC, whose tumors expressed PD-L1 and who received Tecentriq plus nab-paclitaxel. Results from the phase III IMpassion131 study, assessing Tecentriq in combination with paclitaxel for treating people with metastatic TNBC and whose tumors expressed PD-L1, did not meet the trial’s primary endpoint of progression-free survival.
Gavreto was the recipient of U.S. marketing approval during September for the treatment of adults with metastatic rearranged during transfection (RET) fusion-positive non-small cell lung cancer. Composed of the active chemical pralsetinib, the once-daily, oral precision therapy selectively inhibits RET-altered cancers. Gavreto also garnered FDA Priority Review for treating people with advanced or metastatic RET-mutant medullary thyroid cancer and RET fusion-positive thyroid cancer.
Genentech and Blueprint Medicines are joint commercialization partners for pralsetinib in the United States via a deal announced in July. Roche obtained co-development and co-commercialization rights for the investigational medicine, which is also in late-stage development for various types of thyroid cancer and other solid tumors. Roche is additionally responsible for commercial activities outside the United States, excluding Greater China.
U.S. health regulators granted approval in August to Evrysdi (risdiplam) for the treatment of spinal muscular atrophy (SMA) in adults and children 2 months and older. Evrysdi represents the first medicine for SMA that can be administered at home. In two clinical studies, the medicine improved motor function in people living with SMA over a broad spectrum of ages and levels of disease severity, including Types 1, 2, and 3 SMA. Evrysdi helped infants survive without permanent ventilation and achieve the ability to sit without support, regarded as a key motor milestone not typically seen in the natural course of the disease.
Roche unveiled in late September new 2-year data from Part 1 of the pivotal FIREFISH study of Evrysdi in infants aged 2-7 months with symptomatic Type 1 SMA. The 2-year results in infants treated with the therapeutic dose of Evrysdi (17/21) demonstrated that they continued to improve and achieve motor milestones. Exploratory efficacy data demonstrated 88% of infants treated with Evrysdi were alive and did not require permanent ventilation at two years, and 59% of infants were able to sit without support for at least 5 seconds.
Data unveiled in June of an exploratory efficacy analysis from Part 1 of the pivotal SUNFISH study in people aged 2-25 years with type 2 or 3 SMA demonstrate that risdiplam significantly improved motor function after 24 months of treatment compared to natural history data. Also, preliminary 12-month data from JEWELFISH – a study in people with all types of SMA aged 6 months to 60 years previously treated with other SMA therapies – demonstrated that treatment with risdiplam resulted in rapid and sustained increases in SMN protein levels.
One-year data reported in April from FIREFISH Part 2 demonstrate that the study met the primary endpoint with 29% of infants sitting without support for five seconds by month 12, as assessed by the Gross Motor Scale of the Bayley Scales of Infant and Toddler Development Third Edition.
Enspryng (satralizumab-mwge) won marketing clearance in August as the first FDA-approved subcutaneous treatment option for anti-aquaporin-4 (AQP4) antibody positive neuromyelitis optica spectrum disorder (NMOSD) that can be self-administered by a person with NMOSD or a caregiver every four weeks. Enspryng is additionally the first approved therapy for NMOSD designed to target and inhibit interleukin-6 receptor activity, using novel recycling antibody technology. The U.S. approval was supported by one of the largest clinical study programs undertaken for this rare disease.
Enspryng was approved for marketing in Japan during June for the prevention of relapses of NMOSD, including neuromyelitis optica (NMO), for AQP4-IgG seropositive adults and children. Enspryng showed robust efficacy and significantly reduced the risk of relapse across a broad NMOSD patient population in two pivotal phase III trials, as a monotherapy and as an add-on therapy to baseline immunosuppressant therapy (IST). The product is dosed subcutaneously every four weeks. Enspryng is additionally approved in Canada and Switzerland.
Roche reported new data in September showing Enspryng significantly reduces severity and risk of relapse in NMOSD. Enspryng lowered relapse severity in double-blind periods of the SAkura phase III trials. Pooled data from SAkura open-label extension (OLE) trials support continued effect of the product in reducing risk of relapse in the longer term. Roche says ongoing data continues to demonstrate a favorable safety profile.
The FDA granted approval in June for Phesgo – a fixed-dose combination of Perjeta (pertuzumab) and Herceptin (trastuzumab) with hyaluronidase, administered by subcutaneous injection (SC) in combination with intravenous chemotherapy – for the treatment of early and metastatic HER2-positive breast cancer. This is the first time that Roche has combined two monoclonal antibodies that can be administered via one SC injection.
Rozlytrek, Roche’s first tumor-agnostic therapy, was cleared for marketing in Europe during August for people with NTRK fusion-positive solid tumors and for people with ROS1-positive advanced non-small cell lung cancer. Roche management says this approval demonstrates the value of combining genomic profiling with precision medicine to offer patients with rare and hard-to-treat cancers a personalized treatment option. The medicine has demonstrated durable responses across multiple tumor types, including cancer that has spread to the brain.
The EMA granted approval during May for a new, shorter two-hour Ocrevus (ocrelizumab) infusion time – dosed twice yearly – for relapsing or primary progressive multiple sclerosis (MS). Roche says the marketing clearance will further improve the treatment experience for patients while increasing capacity in healthcare systems. The U.S. FDA and EMA accepted applications for a shorter 2-hour infusion time of Ocrevus in April.
New data unveiled in September further reinforce Ocrevus as a highly effective treatment for people with MS. The new data demonstrate that Ocrevus is a highly effective treatment option for people with relapsing-remitting multiple sclerosis (RRMS) who experienced a suboptimal response to their prior disease modifying therapy (DMT). Subgroup analysis from the two-year open-label phase IIIb CASTING trial additionally shows that patients benefit across a wide range of disease-related and demographic subgroups, regardless of prior treatment background.
Earlier in September, Roche initiated novel clinical studies for Ocrevus. The phase IIIb clinical trial program of higher-dose Ocrevus is investigating the impact on reducing disability progression in relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). Also, Roche’s Ocrevus CHIMES study is exclusively focused on disease insights and more tailored care for minority populations with MS.
The European Commission granted marketing clearance in March for Venclyxto plus Gazyvaro for adults with previously untreated chronic lymphocytic leukemia (CLL). The combination regimen provides a new 12-month, fixed-duration, chemotherapy-free treatment option for adult patients with CLL. Data demonstrated that a fixed duration of treatment of Venclyxto with Gazyvaro reduced the risk of disease progression or death by 65% versus a current standard-of-care.
Results from the phase III Viale-A trial reported in June demonstrated that the Venclexta/Venclyxto combination reduced the risk of death (overall survival) by 34% versus azacitidine alone in people with previously untreated acute myeloid leukemia. Venclexta/Venclyxto plus azacitidine also led to higher rates of composite complete remission (CR + CR with incomplete blood count recovery [CR + CRi]) at 66.4% versus 28.3% with azacitidine alone.
The European Commission granted conditional marketing authorization in January for Polivy (polatuzumab vedotin), in combination with bendamustine plus MabThera (rituximab) (BR) for treating adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are not candidates for a hematopoietic stem cell transplant. The first-in-class antibody-drug conjugate Polivy specifically targets the CD79b protein expressed specifically in the majority of B-cells.
In September, a phase III clinical trial program was initiated for the investigational medicine fenebrutinib, designed to be a highly selective and reversible Bruton’s tyrosine kinase (BTK) inhibitor, in RMS and PPMS.
Roche reported in August that etrolizumab met the primary endpoint in phase III trials of inducing remission versus placebo for people with ulcerative colitis in only two of three studies. The new drug candidate failed to meet the primary endpoint versus placebo as maintenance therapy in people with ulcerative colitis. A pivotal phase III trial of etrolizumab in Crohn’s disease continues. Roche is studying other investigational medicines in inflammatory bowel diseases.
The phase III IMpassion031 trial met the study’s primary endpoint by showing a statistically significant and clinically meaningful improvement in pathological complete response (pCR) for treating people with early triple-negative breast cancer (eTNBC), regardless of PD-L1 expression. The clinical trial is assessing Tecentriq in combination with chemotherapy (Abraxane, albumin-bound paclitaxel; nab-paclitaxel; followed by doxorubicin and cyclophosphamide) in comparison with placebo plus chemotherapy (including Abraxane).
Post-hoc analysis from six years of phase III open-label extension trials demonstrated that Ocrevus treatment reduced the risk of needing a walking aid by 49% in RMS patients compared with patients who switched from interferon beta-1a two years later. Separate analysis showed that Ocrevus slowed thalamic volume loss in patients with RMS and PPMS compared to interferon beta-1a and placebo
Updated data reported in May from the pivotal phase III ALEX trial show an increased five-year survival rate with Alecensa (alectinib), versus crizotinib, in people living with anaplastic lymphoma kinase (ALK)-positive NSCLC. Roche says these data confirm the longer-term efficacy of Alecensa already demonstrated across three phase III studies.
Roche reported positive topline results in May from the phase III ARCHWAY trial, assessing the company’s Port Delivery System with ranibizumab (PDS) in people living with neovascular or “wet” age-related macular degeneration (nAMD). PDS is a permanent refillable eye implant the size of a grain of rice, which continuously delivers a customized formulation of ranibizumab (marketed by Roche as Lucentis) during a period of months. The ARCHWAY study met the trial’s primary endpoint, showing that patients with PDS who received refills every six months achieved visual acuity outcomes equivalent to those receiving monthly ranibizumab 0.5 mg injections.
The phase III IPATential150 trial in patients with metastatic castration-resistant prostate cancer and whose tumors had PTEN loss met the co-primary endpoint of radiographic progression-free survival (rPFS). In this patient population, ipatasertib combined with abiraterone and prednisone/prednisolone provided a statistically significant reduction in the risk of disease worsening or death, versus current standard of care (abiraterone and prednisone/ prednisolone) plus placebo. The other co-primary endpoint, rPFS in the overall trial population, was not met as reported in June.
The Investigational CD20xCD3 T-cell engaging bispecific mosunetuzumab was granted FDA Breakthrough Therapy Designation for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma who have received at least two prior systemic therapies. This FDA designation was granted based on encouraging efficacy results observed in the phase I/Ib GO29781 trial studying mosunetuzumab in R/R non- Hodgkin lymphoma.
Diagnostics Approvals/Launches During 2020
FDA Emergency Use Authorization was granted in September for Roche’s cobas SARS-CoV-2 & Influenza A/B Test for use on the cobas 6800/8800 Systems. This represents the first commercial test for fully automated high throughput systems to detect and differentiate SARS-CoV-2, influenza A virus and/or influenza B virus with a single sample. This test is additionally available in markets accepting the CE mark.
Roche received FDA clearance in September for the first HIV-1/HIV-2 Qualitative Test on the cobas 6800/8800 Systems in the fight against HIV/AIDS. The test provides healthcare professionals with a single result to confirm HIV diagnosis and differentiate HIV-1 and HIV-2, a significant distinction necessary to identify appropriate treatment options, according to Roche.
Roche received FDA approval in September for expanded use of the CINtec PLUS Cytology test to aid clinicians in preventing cervical cancer. The new indication allows this first FDA-cleared biomarker-based test to be used as triage for positive cobas human papillomavirus (HPV) tests run on cobas 6800/8800 Systems in primary screening or co-testing programs. The next-generation cytology test was initially approved in March for women whose primary cervical cancer screening results are positive for HPV using the cobas 4800 HPV Test. In April, Roche was granted FDA clearance for the cobas HPV test for use on the cobas 6800/8800 Systems to identify women at risk for cervical cancer.
Another FDA clearance received by Roche in September was for the BK virus quantitative test on cobas 6800/8800 Systems to support better care for transplant patients. The new Breakthrough Device test expands the company’s molecular test menu for transplant patients, enabling simultaneous testing of BK virus with cytomegalovirus and Epstein-Barr virus. On Aug. 5, Roche announced FDA authorization for the first Epstein-Barr virus quantitative test on the cobas 6800/8800 Systems to improve care for transplant patients.
FoundationOne Liquid CDx, a comprehensive pan-tumor liquid biopsy test, was granted U.S. marketing approval as announced by Roche in late August. The company says the test analyzes more than 300 cancer-related genes and multiple genomic signatures to help inform treatment decisions for all solid tumor cancers. Using a simple blood draw, FoundationOne Liquid CD enables more patients with advanced cancer to benefit from the insights of comprehensive genomic profiling, for example when a tissue biopsy is not possible or recommended.
During late July, the FDA approved Roche’s new VENTANA HER2 Dual ISH DNA Probe Cocktail assay as a companion diagnostic to identify breast cancer patients eligible for targeted therapy. The VENTANA HER2 Dual ISH test assists in identifying HER2-positive breast cancer patients eligible for the targeted drug Herceptin. The test was developed with enhanced technology and provides high-quality staining with improved turnaround time, according to Roche.
Roche’s Diagnostics business had a busy second-quarter 2020 with the following launches: several tests for COVID-19 diagnosis; cobas prime, a pre-analytical system for automation in molecular labs; and digital pathology algorithms for non-small cell lung cancer and breast cancer.
Stratos Genomics, an early-stage sequencing technology company, was acquired in May to advance the development of Roche’s nanopore sequencer. The acquisition provides Roche access to Stratos Genomics’ unique chemistry, Sequencing by Expansion. The Roche nanopore sequencer, once developed, will use a novel approach that unites electronic and biological components to sequence DNA for fast, flexible and cost-effective clinical diagnostic testing.
In addition to the COVID-19 portfolio, Roche launched the cobas prime Pre-analytical System. The first- of-its kind solution is designed to automate all common pre-analytical manual steps in molecular diagnostics laboratories. Roche says the system accommodates multiple sample types, simplifies workflow and reduces manual errors. Roche is the first company to offer molecular labs with complete end-to-end automation for testing consolidation on current and future platforms.
Government
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
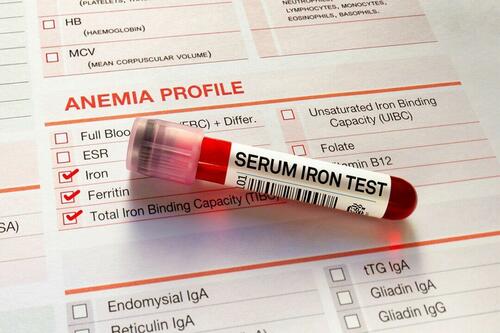
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Government
Walmart joins Costco in sharing key pricing news
The massive retailers have both shared information that some retailers keep very close to the vest.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trumpGovernment
Walmart has really good news for shoppers (and Joe Biden)
The giant retailer joins Costco in making a statement that has political overtones, even if that’s not the intent.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trump-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges



















