Carisma’s CAR-M steals spotlight among 42 bidders to reverse merge with Sesen
Sesen Bio’s decade-plus journey is over after failing to secure a bladder cancer drug approval and carve its own path for IL-6 antibodies. The Cambridge,…

Sesen Bio’s decade-plus journey is over after failing to secure a bladder cancer drug approval and carve its own path for IL-6 antibodies. The Cambridge, MA, company’s spot on Nasdaq will serve as the new home for Carisma Therapeutics, which is attempting to usher in a new CAR cell therapy in the world of macrophages and monocytes.
The two are executing an all-stock reverse merger, with the future resting solely on Carisma’s lineup of therapies, including a Phase I asset that had some limited data presented at ASCO in June. The Philadelphia startup will begin trading under the aptly symboled ticker $CARM in three to four months, the companies said Wednesday morning.
Carisma beat out 41 other bidders, Sesen CEO and president Thomas Cannell said in a statement, noting the merger partner “has the science and the unwavering patient focus required” to better the lives of cancer patients. The Penn-founded startup has also lined up its own investors to bankroll the new Carisma, with $30 million from AbbVie, Merck’s therapeutics VC arm, Penn Medicine and a host of other backers.
In all, Carisma will have $180 million to bring multiple therapies to clinical readouts and keep the lights on through 2024, the companies said. The first program, CT-0508, entails going after advanced HER2+ solid tumors with a CAR macrophage cell therapy. Novartis is picking up the tech to help manufacture the clinical asset.
Next in line is a HER2 CAR monocyte, which CSO and co-founder Michael Klichinsky previously characterized as the precursor to the macrophage. And there’s the in vivo cancer cell therapy pact with Moderna, which included $45 million upfront.
Carisma is taking over the stock listing of an embattled Sesen, which has been in rough waters since before the bear market. Sesen paused the development of its bladder cancer drug in July, after a 2021 FDA rejection and multiple meetings thereafter, and slashed its workforce. The company also fully offloaded its IL-6 antibodies to Roche for $40 million upfront and $30 million more on the line.
Sesen Bio stockholders will own about 41.7% of the merged company, and Carisma stockholders will get the remaining 58.3%. Carisma will remain in Philadelphia and keep CEO and president Steven Kelly at the helm.
nasdaq covid-19 fda antibodies therapyUncategorized
Comments on February Employment Report
The headline jobs number in the February employment report was above expectations; however, December and January payrolls were revised down by 167,000 combined. The participation rate was unchanged, the employment population ratio decreased, and the …
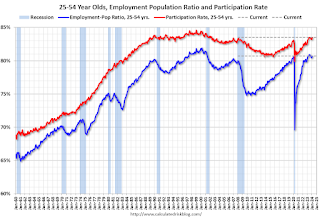
Prime (25 to 54 Years Old) Participation
Since the overall participation rate is impacted by both cyclical (recession) and demographic (aging population, younger people staying in school) reasons, here is the employment-population ratio for the key working age group: 25 to 54 years old.
The 25 to 54 years old participation rate increased in February to 83.5% from 83.3% in January, and the 25 to 54 employment population ratio increased to 80.7% from 80.6% the previous month.
Average Hourly Wages
 The graph shows the nominal year-over-year change in "Average Hourly Earnings" for all private employees from the Current Employment Statistics (CES).
The graph shows the nominal year-over-year change in "Average Hourly Earnings" for all private employees from the Current Employment Statistics (CES). Wage growth has trended down after peaking at 5.9% YoY in March 2022 and was at 4.3% YoY in February.
Part Time for Economic Reasons
 From the BLS report:
From the BLS report:"The number of people employed part time for economic reasons, at 4.4 million, changed little in February. These individuals, who would have preferred full-time employment, were working part time because their hours had been reduced or they were unable to find full-time jobs."The number of persons working part time for economic reasons decreased in February to 4.36 million from 4.42 million in February. This is slightly above pre-pandemic levels.
These workers are included in the alternate measure of labor underutilization (U-6) that increased to 7.3% from 7.2% in the previous month. This is down from the record high in April 2020 of 23.0% and up from the lowest level on record (seasonally adjusted) in December 2022 (6.5%). (This series started in 1994). This measure is above the 7.0% level in February 2020 (pre-pandemic).
Unemployed over 26 Weeks
 This graph shows the number of workers unemployed for 27 weeks or more.
This graph shows the number of workers unemployed for 27 weeks or more. According to the BLS, there are 1.203 million workers who have been unemployed for more than 26 weeks and still want a job, down from 1.277 million the previous month.
This is close to pre-pandemic levels.
Job Streak
| Headline Jobs, Top 10 Streaks | ||
|---|---|---|
| Year Ended | Streak, Months | |
| 1 | 2019 | 100 |
| 2 | 1990 | 48 |
| 3 | 2007 | 46 |
| 4 | 1979 | 45 |
| 5 | 20241 | 38 |
| 6 tie | 1943 | 33 |
| 6 tie | 1986 | 33 |
| 6 tie | 2000 | 33 |
| 9 | 1967 | 29 |
| 10 | 1995 | 25 |
| 1Currrent Streak | ||
Summary:
The headline monthly jobs number was above consensus expectations; however, December and January payrolls were revised down by 167,000 combined. The participation rate was unchanged, the employment population ratio decreased, and the unemployment rate was increased to 3.9%. Another solid report.
Uncategorized
Immune cells can adapt to invading pathogens, deciding whether to fight now or prepare for the next battle
When faced with a threat, T cells have the decision-making flexibility to both clear out the pathogen now and ready themselves for a future encounter.
How does your immune system decide between fighting invading pathogens now or preparing to fight them in the future? Turns out, it can change its mind.
Every person has 10 million to 100 million unique T cells that have a critical job in the immune system: patrolling the body for invading pathogens or cancerous cells to eliminate. Each of these T cells has a unique receptor that allows it to recognize foreign proteins on the surface of infected or cancerous cells. When the right T cell encounters the right protein, it rapidly forms many copies of itself to destroy the offending pathogen.

Importantly, this process of proliferation gives rise to both short-lived effector T cells that shut down the immediate pathogen attack and long-lived memory T cells that provide protection against future attacks. But how do T cells decide whether to form cells that kill pathogens now or protect against future infections?
We are a team of bioengineers studying how immune cells mature. In our recently published research, we found that having multiple pathways to decide whether to kill pathogens now or prepare for future invaders boosts the immune system’s ability to effectively respond to different types of challenges.
Fight or remember?
To understand when and how T cells decide to become effector cells that kill pathogens or memory cells that prepare for future infections, we took movies of T cells dividing in response to a stimulus mimicking an encounter with a pathogen.
Specifically, we tracked the activity of a gene called T cell factor 1, or TCF1. This gene is essential for the longevity of memory cells. We found that stochastic, or probabilistic, silencing of the TCF1 gene when cells confront invading pathogens and inflammation drives an early decision between whether T cells become effector or memory cells. Exposure to higher levels of pathogens or inflammation increases the probability of forming effector cells.
Surprisingly, though, we found that some effector cells that had turned off TCF1 early on were able to turn it back on after clearing the pathogen, later becoming memory cells.
Through mathematical modeling, we determined that this flexibility in decision making among memory T cells is critical to generating the right number of cells that respond immediately and cells that prepare for the future, appropriate to the severity of the infection.
Understanding immune memory
The proper formation of persistent, long-lived T cell memory is critical to a person’s ability to fend off diseases ranging from the common cold to COVID-19 to cancer.
From a social and cognitive science perspective, flexibility allows people to adapt and respond optimally to uncertain and dynamic environments. Similarly, for immune cells responding to a pathogen, flexibility in decision making around whether to become memory cells may enable greater responsiveness to an evolving immune challenge.
Memory cells can be subclassified into different types with distinct features and roles in protective immunity. It’s possible that the pathway where memory cells diverge from effector cells early on and the pathway where memory cells form from effector cells later on give rise to particular subtypes of memory cells.
Our study focuses on T cell memory in the context of acute infections the immune system can successfully clear in days, such as cold, the flu or food poisoning. In contrast, chronic conditions such as HIV and cancer require persistent immune responses; long-lived, memory-like cells are critical for this persistence. Our team is investigating whether flexible memory decision making also applies to chronic conditions and whether we can leverage that flexibility to improve cancer immunotherapy.
Resolving uncertainty surrounding how and when memory cells form could help improve vaccine design and therapies that boost the immune system’s ability to provide long-term protection against diverse infectious diseases.
Kathleen Abadie was funded by a NSF (National Science Foundation) Graduate Research Fellowships. She performed this research in affiliation with the University of Washington Department of Bioengineering.
Elisa Clark performed her research in affiliation with the University of Washington (UW) Department of Bioengineering and was funded by a National Science Foundation Graduate Research Fellowship (NSF-GRFP) and by a predoctoral fellowship through the UW Institute for Stem Cell and Regenerative Medicine (ISCRM).
Hao Yuan Kueh receives funding from the National Institutes of Health.
stimulus covid-19 yuan vaccine stimulusInternational
President Biden Delivers The “Darkest, Most Un-American Speech Given By A President”
President Biden Delivers The "Darkest, Most Un-American Speech Given By A President"
Having successfully raged, ranted, lied, and yelled through…

Having successfully raged, ranted, lied, and yelled through the State of The Union, President Biden can go back to his crypt now.
Whatever 'they' gave Biden, every American man, woman, and the other should be allowed to take it - though it seems the cocktail brings out 'dark Brandon'?
Tl;dw: Biden's Speech tonight ...
-
Fund Ukraine.
-
Trump is threat to democracy and America itself.
-
Abortion is good.
-
American Economy is stronger than ever.
-
Inflation wasn't Biden's fault.
-
Illegals are Americans too.
-
Republicans are responsible for the border crisis.
-
Trump is bad.
-
Biden stands with trans-children.
-
J6 was the worst insurrection since the Civil War.
(h/t @TCDMS99)
Tucker Carlson's response sums it all up perfectly:
"that was possibly the darkest, most un-American speech given by an American president. It wasn't a speech, it was a rant..."
Carlson continued: "The true measure of a nation's greatness lies within its capacity to control borders, yet Bid refuses to do it."
"In a fair election, Joe Biden cannot win"
And concluded:
“There was not a meaningful word for the entire duration about the things that actually matter to people who live here.”
Victor Davis Hanson added some excellent color, but this was probably the best line on Biden:
"he doesn't care... he lives in an alternative reality."
— Tucker Carlson (@TuckerCarlson) March 8, 2024
* * *
Watch SOTU Live here...
* * *
Mises' Connor O'Keeffe, warns: "Be on the Lookout for These Lies in Biden's State of the Union Address."
On Thursday evening, President Joe Biden is set to give his third State of the Union address. The political press has been buzzing with speculation over what the president will say. That speculation, however, is focused more on how Biden will perform, and which issues he will prioritize. Much of the speech is expected to be familiar.
The story Biden will tell about what he has done as president and where the country finds itself as a result will be the same dishonest story he's been telling since at least the summer.
He'll cite government statistics to say the economy is growing, unemployment is low, and inflation is down.
Something that has been frustrating Biden, his team, and his allies in the media is that the American people do not feel as economically well off as the official data says they are. Despite what the White House and establishment-friendly journalists say, the problem lies with the data, not the American people's ability to perceive their own well-being.
As I wrote back in January, the reason for the discrepancy is the lack of distinction made between private economic activity and government spending in the most frequently cited economic indicators. There is an important difference between the two:
-
Government, unlike any other entity in the economy, can simply take money and resources from others to spend on things and hire people. Whether or not the spending brings people value is irrelevant
-
It's the private sector that's responsible for producing goods and services that actually meet people's needs and wants. So, the private components of the economy have the most significant effect on people's economic well-being.
Recently, government spending and hiring has accounted for a larger than normal share of both economic activity and employment. This means the government is propping up these traditional measures, making the economy appear better than it actually is. Also, many of the jobs Biden and his allies take credit for creating will quickly go away once it becomes clear that consumers don't actually want whatever the government encouraged these companies to produce.
On top of all that, the administration is dealing with the consequences of their chosen inflation rhetoric.
Since its peak in the summer of 2022, the president's team has talked about inflation "coming back down," which can easily give the impression that it's prices that will eventually come back down.
But that's not what that phrase means. It would be more honest to say that price increases are slowing down.
Americans are finally waking up to the fact that the cost of living will not return to prepandemic levels, and they're not happy about it.
The president has made some clumsy attempts at damage control, such as a Super Bowl Sunday video attacking food companies for "shrinkflation"—selling smaller portions at the same price instead of simply raising prices.
In his speech Thursday, Biden is expected to play up his desire to crack down on the "corporate greed" he's blaming for high prices.
In the name of "bringing down costs for Americans," the administration wants to implement targeted price ceilings - something anyone who has taken even a single economics class could tell you does more harm than good. Biden would never place the blame for the dramatic price increases we've experienced during his term where it actually belongs—on all the government spending that he and President Donald Trump oversaw during the pandemic, funded by the creation of $6 trillion out of thin air - because that kind of spending is precisely what he hopes to kick back up in a second term.
If reelected, the president wants to "revive" parts of his so-called Build Back Better agenda, which he tried and failed to pass in his first year. That would bring a significant expansion of domestic spending. And Biden remains committed to the idea that Americans must be forced to continue funding the war in Ukraine. That's another topic Biden is expected to highlight in the State of the Union, likely accompanied by the lie that Ukraine spending is good for the American economy. It isn't.
It's not possible to predict all the ways President Biden will exaggerate, mislead, and outright lie in his speech on Thursday. But we can be sure of two things. The "state of the Union" is not as strong as Biden will say it is. And his policy ambitions risk making it much worse.
* * *
The American people will be tuning in on their smartphones, laptops, and televisions on Thursday evening to see if 'sloppy joe' 81-year-old President Joe Biden can coherently put together more than two sentences (even with a teleprompter) as he gives his third State of the Union in front of a divided Congress.
President Biden will speak on various topics to convince voters why he shouldn't be sent to a retirement home.
The state of our union under President Biden: three years of decline. pic.twitter.com/Da1KOIb3eR
— Speaker Mike Johnson (@SpeakerJohnson) March 7, 2024
According to CNN sources, here are some of the topics Biden will discuss tonight:
Economic issues: Biden and his team have been drafting a speech heavy on economic populism, aides said, with calls for higher taxes on corporations and the wealthy – an attempt to draw a sharp contrast with Republicans and their likely presidential nominee, Donald Trump.
Health care expenses: Biden will also push for lowering health care costs and discuss his efforts to go after drug manufacturers to lower the cost of prescription medications — all issues his advisers believe can help buoy what have been sagging economic approval ratings.
Israel's war with Hamas: Also looming large over Biden's primetime address is the ongoing Israel-Hamas war, which has consumed much of the president's time and attention over the past few months. The president's top national security advisers have been working around the clock to try to finalize a ceasefire-hostages release deal by Ramadan, the Muslim holy month that begins next week.
An argument for reelection: Aides view Thursday's speech as a critical opportunity for the president to tout his accomplishments in office and lay out his plans for another four years in the nation's top job. Even though viewership has declined over the years, the yearly speech reliably draws tens of millions of households.
Sources provided more color on Biden's SOTU address:
The speech is expected to be heavy on economic populism. The president will talk about raising taxes on corporations and the wealthy. He'll highlight efforts to cut costs for the American people, including pushing Congress to help make prescription drugs more affordable.
Biden will talk about the need to preserve democracy and freedom, a cornerstone of his re-election bid. That includes protecting and bolstering reproductive rights, an issue Democrats believe will energize voters in November. Biden is also expected to promote his unity agenda, a key feature of each of his addresses to Congress while in office.
Biden is also expected to give remarks on border security while the invasion of illegals has become one of the most heated topics among American voters. A majority of voters are frustrated with radical progressives in the White House facilitating the illegal migrant invasion.
It is probable that the president will attribute the failure of the Senate border bill to the Republicans, a claim many voters view as unfounded. This is because the White House has the option to issue an executive order to restore border security, yet opts not to do so
Maybe this is why?
Most Americans are still unaware that the census counts ALL people, including illegal immigrants, for deciding how many House seats each state gets!
— Elon Musk (@elonmusk) March 7, 2024
This results in Dem states getting roughly 20 more House seats, which is another strong incentive for them not to deport illegals.
While Biden addresses the nation, the Biden administration will be armed with a social media team to pump propaganda to at least 100 million Americans.
"The White House hosted about 70 creators, digital publishers, and influencers across three separate events" on Wednesday and Thursday, a White House official told CNN.
Not a very capable social media team...
The State of Confusion https://t.co/C31mHc5ABJ
— zerohedge (@zerohedge) March 7, 2024
The administration's move to ramp up social media operations comes as users on X are mostly free from government censorship with Elon Musk at the helm. This infuriates Democrats, who can no longer censor their political enemies on X.
Meanwhile, Democratic lawmakers tell Axios that the president's SOTU performance will be critical as he tries to dispel voter concerns about his elderly age. The address reached as many as 27 million people in 2023.
"We are all nervous," said one House Democrat, citing concerns about the president's "ability to speak without blowing things."
The SOTU address comes as Biden's polling data is in the dumps.
BetOnline has created several money-making opportunities for gamblers tonight, such as betting on what word Biden mentions the most.
As well as...
We will update you when Tucker Carlson's live feed of SOTU is published.
Fuck it. We’ll do it live! Thursday night, March 7, our live response to Joe Biden’s State of the Union speech. pic.twitter.com/V0UwOrgKvz
— Tucker Carlson (@TuckerCarlson) March 6, 2024
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 month ago
International1 month agoWar Delirium
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges
























