Uncategorized
Brii Biosciences Provides Update on Strategic Clinical Development Progress
Brii Biosciences Provides Update on Strategic Clinical Development Progress
PR Newswire
DURHAM, N.C., and BEIJING, Dec. 27, 2022
Topline Phase 2 data from multiple hepatitis B viral (HBV) infection functional cure combination studies expected in th…

Brii Biosciences Provides Update on Strategic Clinical Development Progress
PR Newswire
DURHAM, N.C., and BEIJING, Dec. 27, 2022
Topline Phase 2 data from multiple hepatitis B viral (HBV) infection functional cure combination studies expected in the first half of 2023
Initiation of Phase 2 postpartum depression (PPD) study planned for early 2023 to advance potential first-of-its-kind treatment
U.S. FDA lifted clinical hold on BRII-732 allowing the Company to reinitiate a clinical trial to investigate a lower once-weekly oral dose, intended for the treatment and prevention of HIV infection
DURHAM, N.C., and BEIJING, Dec. 27, 2022 /PRNewswire/ -- Brii Biosciences Limited ("Brii Bio" or the "Company", stock code: 2137.HK), a biotechnology company developing therapies to improve patient health and choice across diseases with high unmet need, today announced progress against its strategic clinical development priorities, including various updates across its pipeline of infectious disease and central nervous system disease candidates.
"We continue to progress clinical development of our two lead pipeline programs for a novel functional cure for hepatitis B viral infection in China and a potential first-of-its-kind treatment for postpartum depression in the U.S.," said Zhi Hong, Ph.D., Chairman and Chief Executive Officer of Brii Bio. "These recent advancements in HBV and PPD provide a sharper focus for our China and U.S. teams, respectively, and further exemplify our differentiated approach to pursue curative or single treatment options in two large and important disease areas. We look forward to continuing to foster strategic collaborations and harness our proven development capabilities as we advance our pipeline of therapeutic candidates for patients."
Updates to Brii Bio's Lead Clinical Programs
Hepatitis B Virus (HBV)
Brii Bio is building a novel and first-in-class clinical portfolio of HBV therapeutic candidates alongside our strategic partners that may be used in various combinations to improve the probability of achieving a high rate of functional cure for each subpopulation of HBV patients in China. While HBV is one of the world's most significant infectious disease threats, China has the largest prevalence of HBV in the world, with 87 million people impacted by this disease.
- Patients have completed treatment in Brii Bio's ongoing Phase 2 multi-regional clinical trial (MRCT) combination study of BRII-179 (VBI-2601) and BRII-835 (VIR-2218). The abstract highlighting the interim safety and efficacy data from this study has been accepted for oral presentation at the 32nd Conference of the Asian Pacific Association for the Study of the Liver (APASL), taking place in Taipei, Taiwan, from February 15-19, 2023.
- In December, the Company completed patient enrollment in part one of a Phase 2 combination trial evaluating the addition of BRII-179 (VBI-2601) in chronic HBV patients already receiving pegylated interferon alpha (PEG-IFN-α) and nucleotide/nucleoside reverse transcriptase inhibitors (NRTI) treatment. Topline results are expected in the third quarter of 2023.
- Brii Bio's strategic development partner, Vir Biotechnology, shared preliminary data from an ongoing Phase 2 trial of combination BRII-835 (VIR-2218) with PEG-IFN-α, which demonstrated that approximately 30% of patients with chronic HBV infection achieved HBsAg seroclearance with anti-HBs seroconversion by the end of treatment with no new safety signals. The results were presented in an oral session at the American Association for the Study of Liver Diseases (AASLD) The Liver Meeting®.
- Initial data from Part B of Vir's ongoing Phase 2 MARCH trial are expected in the second half of 2023 and will evaluate BRII-835 (VIR-2218) and BRII-877 (VIR-3434) together and in triple combination with PEG-IFN-α, including determining dose and length of treatment.
Postpartum Depression (PPD)
Brii Bio is developing BRII-296 as a first-of-its-kind one-time injection therapeutic candidate with the potential to expand the PPD treatment landscape for patients in the U.S., where one in seven new mothers are impacted by this condition.
- Brii Bio recently participated in a Type C meeting with the U.S. Food and Drug Administration (FDA) at which it received productive feedback and guidance regarding the Company's Investigational New Drug (IND) application for BRII-296. Following this discussion, Brii Bio is preparing to initiate its Phase 2 study of BRII-296 for the treatment of PPD in early 2023.
- In September, Brii Bio announced positive topline results from its Phase 1 study of BRII-296 with data that demonstrated a single administration of the investigational therapy at 600 mg delivered a favorable pharmacokinetic profile and was safe and well-tolerated in healthy subjects. Furthermore, early feedback from physicians and patient communities are very positive and reinforce the potential for a first-of-its-kind single-injection treatment option for PPD.
Additional Pre-Clinical and Clinical Development Updates
Human Immunodeficiency Virus (HIV) Infection
- Based on pharmacokinetic (PK) data from a completed Phase 1 study, Brii Bio has made the decision to discontinue development of BRII-778, which was being evaluated as part of a potential long-acting combination therapy for HIV infection. The Company is exploring partnership opportunities to continue developing BRII-732 as part of a potential oral, once-weekly, long-acting combination treatment option for HIV-1 patients.
- In October, Brii Bio presented positive Phase 1 data showing that BRII-732 demonstrated an acceptable safety and tolerability profile, as well as a favorable and linear PK profile that achieved therapeutic targets in healthy volunteers, reinforcing its potential as an once-weekly oral therapy for the treatment and prevention of HIV infections.
- Brii Bio recently received notification that the U.S. FDA has lifted the clinical hold on the Company's planned Phase 1 study to investigate a lower oral dose of once-weekly BRII-732.
MDR/XDR Gram-Negative Bacteria Infections
- BRII-672 has the potential to be the first oral treatment option for complicated urinary tract infections (cUTI) targeting gram-negative bacteria resistant to carbapenem. In December 2022, the first pre-IND was submitted to the National Medical Products Administration (NMPA) seeking regulatory guidance around a development plan for BRII-672 in China.
- BRII-693 has a highly differentiated safety and efficacy profile to address the most difficult-to-treat Acinetobacter baumannii and Pseudomonas aeruginosa infections resistant to carbapenem. Brii Bio plans to submit a pre-IND to the China NMPA in the first quarter of 2023.
- Preclinical data and interim Phase 1 clinical results for BRII-636, BRII-672 and BRII-693 were presented at IDWeek in October 2022.
Nontuberculosis Mycobacteria (NTM) Lung Disease
- Brii Bio's strategic partner, AN2 Therapeutics, expects to complete enrollment in the Phase 2 part of the pivotal Phase 2/3 clinical trial evaluating BRII-658 (epetraborole) for treatment-refractory lung disease caused by Mycobacterium avium complex (MAC) in mid-2023 and plans to begin enrollment of the Phase 3 portion of the trial immediately thereafter.
COVID-19
- Following the Company's commercial launch of the amubarvimab/romlusevimab combination in China, the long-acting COVID-19 neutralizing antibody therapy is being used to treat patients in China, and early feedback from physicians has been positive.
- Based on ongoing preclinical testing, the amubarvimab/romlusevimab combination retains neutralizing activity against the major SARS-CoV-2 subvariants currently circulating in China, underscoring the antibody combination as an important treatment option for high risk patients in China.
About Brii Bio
Brii Biosciences is a biotechnology company developing therapies to address some of the world's most common diseases where patients experience high unmet medical needs, limited choice and significant social stigmas. With a focus on infectious and central nervous system diseases, the Company is advancing a broad pipeline of unique therapeutic candidates with lead programs to develop a novel functional cure for hepatitis B viral infection (HBV) and a first-of-its-kind treatment for postpartum depression (PPD). The Company is led by a visionary and experienced leadership team and has operations in key biotech hubs, including Raleigh-Durham, the San Francisco Bay Area, Beijing and Shanghai. For more information, visit www.briibio.com.
View original content to download multimedia:https://www.prnewswire.com/news-releases/brii-biosciences-provides-update-on-strategic-clinical-development-progress-301709926.html
SOURCE Brii Biosciences Limited
Uncategorized
Comments on February Employment Report
The headline jobs number in the February employment report was above expectations; however, December and January payrolls were revised down by 167,000 combined. The participation rate was unchanged, the employment population ratio decreased, and the …
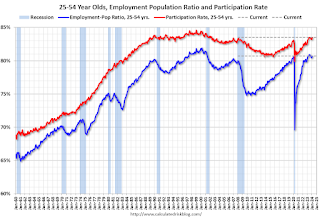
Prime (25 to 54 Years Old) Participation
Since the overall participation rate is impacted by both cyclical (recession) and demographic (aging population, younger people staying in school) reasons, here is the employment-population ratio for the key working age group: 25 to 54 years old.
The 25 to 54 years old participation rate increased in February to 83.5% from 83.3% in January, and the 25 to 54 employment population ratio increased to 80.7% from 80.6% the previous month.
Average Hourly Wages
 The graph shows the nominal year-over-year change in "Average Hourly Earnings" for all private employees from the Current Employment Statistics (CES).
The graph shows the nominal year-over-year change in "Average Hourly Earnings" for all private employees from the Current Employment Statistics (CES). Wage growth has trended down after peaking at 5.9% YoY in March 2022 and was at 4.3% YoY in February.
Part Time for Economic Reasons
 From the BLS report:
From the BLS report:"The number of people employed part time for economic reasons, at 4.4 million, changed little in February. These individuals, who would have preferred full-time employment, were working part time because their hours had been reduced or they were unable to find full-time jobs."The number of persons working part time for economic reasons decreased in February to 4.36 million from 4.42 million in February. This is slightly above pre-pandemic levels.
These workers are included in the alternate measure of labor underutilization (U-6) that increased to 7.3% from 7.2% in the previous month. This is down from the record high in April 2020 of 23.0% and up from the lowest level on record (seasonally adjusted) in December 2022 (6.5%). (This series started in 1994). This measure is above the 7.0% level in February 2020 (pre-pandemic).
Unemployed over 26 Weeks
 This graph shows the number of workers unemployed for 27 weeks or more.
This graph shows the number of workers unemployed for 27 weeks or more. According to the BLS, there are 1.203 million workers who have been unemployed for more than 26 weeks and still want a job, down from 1.277 million the previous month.
This is close to pre-pandemic levels.
Job Streak
| Headline Jobs, Top 10 Streaks | ||
|---|---|---|
| Year Ended | Streak, Months | |
| 1 | 2019 | 100 |
| 2 | 1990 | 48 |
| 3 | 2007 | 46 |
| 4 | 1979 | 45 |
| 5 | 20241 | 38 |
| 6 tie | 1943 | 33 |
| 6 tie | 1986 | 33 |
| 6 tie | 2000 | 33 |
| 9 | 1967 | 29 |
| 10 | 1995 | 25 |
| 1Currrent Streak | ||
Summary:
The headline monthly jobs number was above consensus expectations; however, December and January payrolls were revised down by 167,000 combined. The participation rate was unchanged, the employment population ratio decreased, and the unemployment rate was increased to 3.9%. Another solid report.
Uncategorized
Immune cells can adapt to invading pathogens, deciding whether to fight now or prepare for the next battle
When faced with a threat, T cells have the decision-making flexibility to both clear out the pathogen now and ready themselves for a future encounter.
How does your immune system decide between fighting invading pathogens now or preparing to fight them in the future? Turns out, it can change its mind.
Every person has 10 million to 100 million unique T cells that have a critical job in the immune system: patrolling the body for invading pathogens or cancerous cells to eliminate. Each of these T cells has a unique receptor that allows it to recognize foreign proteins on the surface of infected or cancerous cells. When the right T cell encounters the right protein, it rapidly forms many copies of itself to destroy the offending pathogen.

Importantly, this process of proliferation gives rise to both short-lived effector T cells that shut down the immediate pathogen attack and long-lived memory T cells that provide protection against future attacks. But how do T cells decide whether to form cells that kill pathogens now or protect against future infections?
We are a team of bioengineers studying how immune cells mature. In our recently published research, we found that having multiple pathways to decide whether to kill pathogens now or prepare for future invaders boosts the immune system’s ability to effectively respond to different types of challenges.
Fight or remember?
To understand when and how T cells decide to become effector cells that kill pathogens or memory cells that prepare for future infections, we took movies of T cells dividing in response to a stimulus mimicking an encounter with a pathogen.
Specifically, we tracked the activity of a gene called T cell factor 1, or TCF1. This gene is essential for the longevity of memory cells. We found that stochastic, or probabilistic, silencing of the TCF1 gene when cells confront invading pathogens and inflammation drives an early decision between whether T cells become effector or memory cells. Exposure to higher levels of pathogens or inflammation increases the probability of forming effector cells.
Surprisingly, though, we found that some effector cells that had turned off TCF1 early on were able to turn it back on after clearing the pathogen, later becoming memory cells.
Through mathematical modeling, we determined that this flexibility in decision making among memory T cells is critical to generating the right number of cells that respond immediately and cells that prepare for the future, appropriate to the severity of the infection.
Understanding immune memory
The proper formation of persistent, long-lived T cell memory is critical to a person’s ability to fend off diseases ranging from the common cold to COVID-19 to cancer.
From a social and cognitive science perspective, flexibility allows people to adapt and respond optimally to uncertain and dynamic environments. Similarly, for immune cells responding to a pathogen, flexibility in decision making around whether to become memory cells may enable greater responsiveness to an evolving immune challenge.
Memory cells can be subclassified into different types with distinct features and roles in protective immunity. It’s possible that the pathway where memory cells diverge from effector cells early on and the pathway where memory cells form from effector cells later on give rise to particular subtypes of memory cells.
Our study focuses on T cell memory in the context of acute infections the immune system can successfully clear in days, such as cold, the flu or food poisoning. In contrast, chronic conditions such as HIV and cancer require persistent immune responses; long-lived, memory-like cells are critical for this persistence. Our team is investigating whether flexible memory decision making also applies to chronic conditions and whether we can leverage that flexibility to improve cancer immunotherapy.
Resolving uncertainty surrounding how and when memory cells form could help improve vaccine design and therapies that boost the immune system’s ability to provide long-term protection against diverse infectious diseases.
Kathleen Abadie was funded by a NSF (National Science Foundation) Graduate Research Fellowships. She performed this research in affiliation with the University of Washington Department of Bioengineering.
Elisa Clark performed her research in affiliation with the University of Washington (UW) Department of Bioengineering and was funded by a National Science Foundation Graduate Research Fellowship (NSF-GRFP) and by a predoctoral fellowship through the UW Institute for Stem Cell and Regenerative Medicine (ISCRM).
Hao Yuan Kueh receives funding from the National Institutes of Health.
stimulus covid-19 yuan vaccine stimulusUncategorized
Stock indexes are breaking records and crossing milestones – making many investors feel wealthier
The S&P 500 topped 5,000 on Feb. 9, 2024, for the first time. The Dow Jones Industrial Average will probably hit a new big round number soon t…

The S&P 500 stock index topped 5,000 for the first time on Feb. 9, 2024, exciting some investors and garnering a flurry of media coverage. The Conversation asked Alexander Kurov, a financial markets scholar, to explain what stock indexes are and to say whether this kind of milestone is a big deal or not.
What are stock indexes?
Stock indexes measure the performance of a group of stocks. When prices rise or fall overall for the shares of those companies, so do stock indexes. The number of stocks in those baskets varies, as does the system for how this mix of shares gets updated.
The Dow Jones Industrial Average, also known as the Dow, includes shares in the 30 U.S. companies with the largest market capitalization – meaning the total value of all the stock belonging to shareholders. That list currently spans companies from Apple to Walt Disney Co.
The S&P 500 tracks shares in 500 of the largest U.S. publicly traded companies.
The Nasdaq composite tracks performance of more than 2,500 stocks listed on the Nasdaq stock exchange.
The DJIA, launched on May 26, 1896, is the oldest of these three popular indexes, and it was one of the first established.
Two enterprising journalists, Charles H. Dow and Edward Jones, had created a different index tied to the railroad industry a dozen years earlier. Most of the 12 stocks the DJIA originally included wouldn’t ring many bells today, such as Chicago Gas and National Lead. But one company that only got booted in 2018 had stayed on the list for 120 years: General Electric.
The S&P 500 index was introduced in 1957 because many investors wanted an option that was more representative of the overall U.S. stock market. The Nasdaq composite was launched in 1971.
You can buy shares in an index fund that mirrors a particular index. This approach can diversify your investments and make them less prone to big losses.
Index funds, which have only existed since Vanguard Group founder John Bogle launched the first one in 1976, now hold trillions of dollars .
Why are there so many?
There are hundreds of stock indexes in the world, but only about 50 major ones.
Most of them, including the Nasdaq composite and the S&P 500, are value-weighted. That means stocks with larger market values account for a larger share of the index’s performance.
In addition to these broad-based indexes, there are many less prominent ones. Many of those emphasize a niche by tracking stocks of companies in specific industries like energy or finance.
Do these milestones matter?
Stock prices move constantly in response to corporate, economic and political news, as well as changes in investor psychology. Because company profits will typically grow gradually over time, the market usually fluctuates in the short term, while increasing in value over the long term.
The DJIA first reached 1,000 in November 1972, and it crossed the 10,000 mark on March 29, 1999. On Jan. 22, 2024, it surpassed 38,000 for the first time. Investors and the media will treat the new record set when it gets to another round number – 40,000 – as a milestone.
The S&P 500 index had never hit 5,000 before. But it had already been breaking records for several weeks.
Because there’s a lot of randomness in financial markets, the significance of round-number milestones is mostly psychological. There is no evidence they portend any further gains.
For example, the Nasdaq composite first hit 5,000 on March 10, 2000, at the end of the dot-com bubble.
The index then plunged by almost 80% by October 2002. It took 15 years – until March 3, 2015 – for it return to 5,000.
By mid-February 2024, the Nasdaq composite was nearing its prior record high of 16,057 set on Nov. 19, 2021.
Index milestones matter to the extent they pique investors’ attention and boost market sentiment.
Investors afflicted with a fear of missing out may then invest more in stocks, pushing stock prices to new highs. Chasing after stock trends may destabilize markets by moving prices away from their underlying values.
When a stock index passes a new milestone, investors become more aware of their growing portfolios. Feeling richer can lead them to spend more.
This is called the wealth effect. Many economists believe that the consumption boost that arises in response to a buoyant stock market can make the economy stronger.
Is there a best stock index to follow?
Not really. They all measure somewhat different things and have their own quirks.
For example, the S&P 500 tracks many different industries. However, because it is value-weighted, it’s heavily influenced by only seven stocks with very large market values.
Known as the “Magnificent Seven,” shares in Amazon, Apple, Alphabet, Meta, Microsoft, Nvidia and Tesla now account for over one-fourth of the S&P 500’s value. Nearly all are in the tech sector, and they played a big role in pushing the S&P across the 5,000 mark.
This makes the index more concentrated on a single sector than it appears.
But if you check out several stock indexes rather than just one, you’ll get a good sense of how the market is doing. If they’re all rising quickly or breaking records, that’s a clear sign that the market as a whole is gaining.
Sometimes the smartest thing is to not pay too much attention to any of them.
For example, after hitting record highs on Feb. 19, 2020, the S&P 500 plunged by 34% in just 23 trading days due to concerns about what COVID-19 would do to the economy. But the market rebounded, with stock indexes hitting new milestones and notching new highs by the end of that year.
Panicking in response to short-term market swings would have made investors more likely to sell off their investments in too big a hurry – a move they might have later regretted. This is why I believe advice from the immensely successful investor and fan of stock index funds Warren Buffett is worth heeding.
Buffett, whose stock-selecting prowess has made him one of the world’s 10 richest people, likes to say “Don’t watch the market closely.”
If you’re reading this because stock prices are falling and you’re wondering if you should be worried about that, consider something else Buffett has said: “The light can at any time go from green to red without pausing at yellow.”
And the opposite is true as well.
Alexander Kurov does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
dow jones sp 500 nasdaq stocks covid-19-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 month ago
International1 month agoWar Delirium
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















