Government
“Being A Doctor And Courageous Runs In My Family”: French Scientist Behind Hydroxychloroquine Treatment For COVID-19
"Being A Doctor And Courageous Runs In My Family": French Scientist Behind Hydroxychloroquine Treatment For COVID-19
Authored by Terri Wu…

Authored by Terri Wu via The Epoch Times (emphasis ours),
Didier Raoult, a renowned French microbiologist, announced hydroxychloroquine (HCQ) as an effective cure for COVID-19 on Feb. 25, 2020. The following month, former U.S. President Donald Trump cited his research and called the drug a “game changer.” Since then, Dr. Raoult has been engulfed in a global controversy.
Initially, in March 2020, the Food and Drug Administration (FDA) granted HCQ, a drug widely used against malaria and arthritis, an emergency use authorization (EUA) for treating COVID-19.
However, on May 22, 2020, the Lancet medical journal published a now-retracted study saying that HCQ brought about a much higher risk of death and heart problems in hospitalized COVID-19 patients, citing data from nearly 100,000 patients in over 600 hospitals. The authors noted the mortality rate of the total surveyed population was 11.1 percent, while the death rate was 16 percent and higher for the groups given various HCQ treatments—nearly double that of the control group, which was not given HCQ treatment.
The study preceded a series of decisions by health authorities. In three days, the World Health Organization (WHO) suspended a clinical trial of HCQ for COVID-19. During the same week, France also banned its use on COVID-19 patients, revoking an emergency authorization of HCQ for COVID-19 issued two weeks earlier.
On June 15, 2020, the FDA revoked the EUA of HCQ, citing cardiac adverse events and other side effects. Six months later, the FDA approved EUAs for Pfizer-BioNTech and Moderna COVID-19 vaccines, emergency authorizations contingent on “no adequate, approved, and available alternatives,” according to the FDA announcement.
“Chloroquine or hydroxychloroquine was prescribed 1 billion times in the year of 2006. So it means that if 10 percent of the people died with this, that would have made 100 million people die of heart attack, which is stupid,” Dr. Raoult told The Epoch Times. HCQ is a newer and improved version of chloroquine.
He said he and other experts wrote to the Lancet, saying the study’s findings were impossible. A few months later, the journal retracted the paper. When retracting the article on June 4, 2020, the authors said they could “no longer vouch for the veracity of the primary data sources.” They also apologized for causing “any embarrassment or inconvenience” to the journal and its readers.
A day before the retraction of the paper, the WHO announced it was resuming the clinical trial of HCQ on COVID-19. On June 20, 2020, the U.S. National Institutes of Health (NIH) halted its clinical trial, citing no harm and no benefit of HCQ on COVID-19 patients. A month later, the WHO also discontinued its HCQ treatment arm for COVID-19 for similar reasons. France's ban on using HCQ for COVID-19 patients has not been lifted.
Yet Dr. Raoult persisted in treating COVID-19 patients with HCQ and azithromycin, an antibiotic. On April 4, 2023, he published preliminary results of treating over 30,000 patients between March 2020 and December 2021 at the institute he led until August 2022, the Institut Hospitalo-Universitaire Méditerranée Infection (IHU). He said the fatality rate of those treated with HCQ was at 7 percent, “half of the fatality rate of the deaths of any series of hospitalized patients [who] needed oxygen.” “So it's very efficient,” he added.
In May, French medical bodies called for sanctions against Dr. Raoult for “the largest 'unauthorised' clinical trial ever seen.” Dr. Raoult responded, "There's no trial, only the therapeutical choice by the doctors."
Over the years, HCQ has remained a contentious topic. Because he stood by his statement of HCQ’s effectiveness on COVID-19, Dr. Raoult, an accomplished scientist who received one of the highest honors in France—the prestigious Grand Prix Inserm—in 2010, has been labeled an anti-vaccine conspiracy theorist.
In response, he has maintained that he is not against vaccines, but to him, the equation of risks and benefits doesn’t hold for COVID-19 patients under 50 with low risk, especially children, to elect to take the injections.
He spoke to The Epoch Times while promoting his autobiography at a conference in Saint-Hyacinthe, a small city east of Montreal. The autobiography was published in France in April and in Canada in October. “Being a doctor and courageous runs in my family,” he said.
His father was a military doctor, and his great-grandfather an infectious disease doctor. He was born in French Dakar, today’s Senegal in West Africa, while his father was posted there working on malnutrition. His grandmother ran a resistance network against the Nazis and survived from Ravensbrück, a women’s concentration camp in northern Germany, 50 miles north of Berlin.
“I don’t want to be ashamed to be less courageous. She was deported to Ravensbrück, my God,” he said. “It’s in my family. Sometimes you need to disobey because you think it’s not good to obey some things incompatible with your moral sense.”
He said patients were told not to see doctors and doctors not to see patients during the COVID lockdown. That was “completely stupid” to him. In March 2020, he opened IHU to COVID-19 patients who were willing to receive HCQ treatments, and people queued in winding single-file lines outside his institute to wait for their turns.

To him, countering the mainstream recommendation that HCQ was dangerous for COVID-19 patients was just an outcome of doing his job. “I don’t care about what the others say. I’m just talking about what I’m seeing. It’s not a Christian opinion; I don’t get any opinion.”
He said he was encouraged by a recent U.S. appeals court decision allowing a lawsuit from three doctors against the FDA to proceed. On Sept. 1, a panel of three judges on the 5th U.S. Circuit Court of Appeal in New Orleans revived the case, which alleges the FDA overstepping its authority by running a campaign against ivermectin’s effectiveness in treating COVID-19 patients.
“It was very interesting because I hope it will happen sometime in France—the doctor is in charge of treating the patient. This is his role and his duty. So you can have recommendation information from the government or from the agency, but it cannot replace the responsibility of the doctor who prescribes the treatment,” Dr. Raoult said.
He added that patients should hold the doctor accountable if he didn’t do his job. “But it’s not an agency that should determine what the doctor [should] do.”
The maverick scientist, now 71, said he wasn't aware of any complaints from the 30,000 COVID-19 patients treated at IHU under his leadership. “This means that the people who have never seen the patient in the Ministry of Health don’t know better than we who treated 30,000 people. And I’ve been practicing medicine for 42 years, so I know what I’m doing.”
“Self-esteem is more important than the esteem of others,” he said about one of the themes of his autobiography. “I need to be agreed with myself. This is critical.”
Tanya Du contributed to this report.
Government
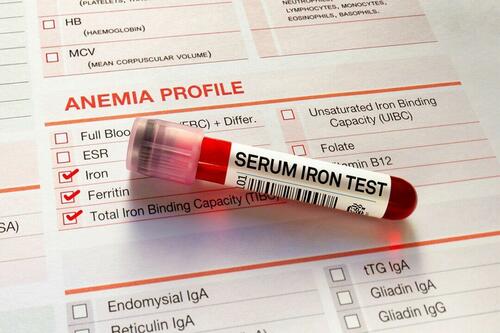
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Government
Walmart joins Costco in sharing key pricing news
The massive retailers have both shared information that some retailers keep very close to the vest.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trumpGovernment
Walmart has really good news for shoppers (and Joe Biden)
The giant retailer joins Costco in making a statement that has political overtones, even if that’s not the intent.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trump-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges



















