The Next Generation of Cell Factories for Viral Vector Production
Although the industry still relies on transient transfection, due to the difficulty of creating stable production cell lines, a shift from adherent to suspension culture is underway.
The post The Next Generation of Cell Factories for Viral Vector Producti
Despite the undeniable ability of viral vectors to deliver genetic payloads, and despite the incredible promise of this approach to treat a vast variety of diseases—including metabolic, cardiovascular, muscular, hematologic, ophthalmologic, and infectious diseases as well as cancer—hurdles remain, especially in manufacturing.
Due to its complexity, viral vector production is still evolving. Experts concur that overall yields of functional capsids containing the desired genetic payload is the biggest obstacle. Most often, transient transfection is used, essentially creating a single-run “cell factory,” that is, a cell line for each manufacturing run. A stable and optimized production cell line, like those used for other biologics, would provide a more robust process, and it would be scalable if cells were grown in suspension.
Progress is being made, but new technologies and approaches to standardize processes take time to develop and implement, especially if the processes involve GMP-grade reagents. To accelerate progress, developers are implementing new tools. Six of these developers are discussed in this article. Each has insights to offer on ways to streamline production.
Scalable host cell lines
Viral vectors are complex—significantly more complex than traditional recombinant protein therapeutics. Besides consisting of both proteins and nucleic acids, viral vectors present daunting production challenges. For example, production processes for viral vectors typically incorporate lipid- or polymer-based transfection reagents and lysis buffers, production components that are not needed in stable, recombinant protein–expressing production systems.
According to Jonathan Zmuda, PhD, director of cell biology, Thermo Fisher Scientific, viral vector production involves challenges both upstream and downstream. Typical challenges include scalability issues, difficulties maintaining productivity, and overall lack of robustness.
Significant progress has been made in recent years to address scalability. Yet the commonly used HEK293 cells remain less well characterized for large-scale production than are their protein expression CHO cell counterparts.
In contrast, Sf9 (Spodoptera frugiperda) insect cells are well validated for cost-effective adeno-associated virus (AAV) production at 1000-L scale or greater; however, insect production systems possess workflow steps with which many laboratories are unfamiliar. Thus, transient transfection of HEK293 cells remains the most efficient way to generate AAV.
“The producer cell line forms the foundation of any viral vector expression system,” says Zmuda. “Great care must be taken to ensure that the appropriate host cell line is chosen at the earliest stages of development.” Shortcomings of the HEK293T adherent platform include scalability and the requirement for animal serum, whereas the presence of the oncogenic large T antigen impacts both adherent and suspension HEK293T platforms.
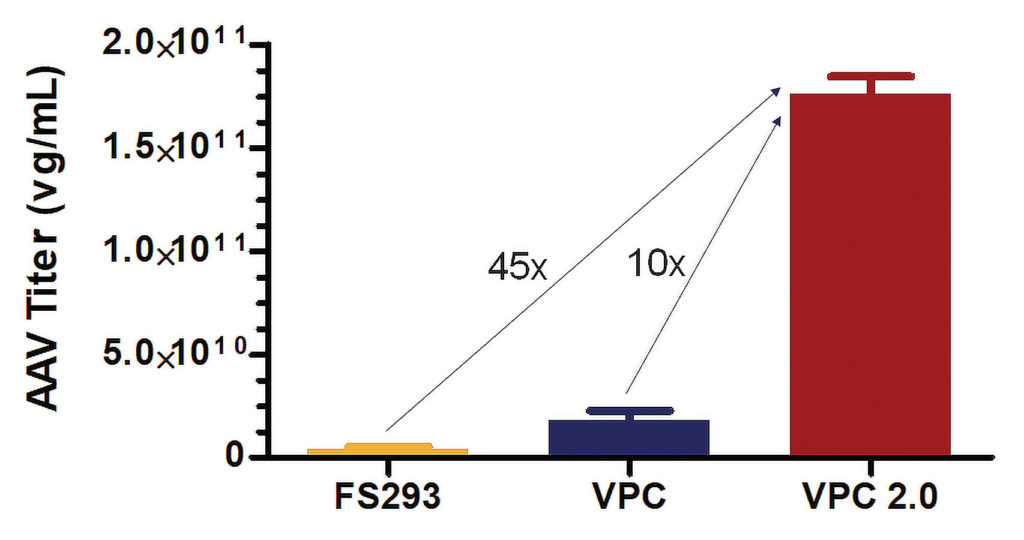
To address the cell line challenges posed by AAV production, Thermo Fisher developed CTS Viral Production Cells (VPCs) for the production of scalable, high-titer lentivirus in suspension HEK293 cells. More recently, Viral Production Cells 2.0 (VPCs 2.0) were developed specifically for AAV production. VPCs 2.0 is a clonal HEK293 cell line selected for its ability to support production of high-titer and high-quality AAV in stirred-tank bioreactors via transient transfection in the Gibco AAV-MAX Helper-Free AAV Production System, which is currently available as a prototype.

Contract development and manufacturing organizations (CDMOs) like Thermo Fisher’s Pharma Services business are a viable option for gene therapy developers that need viral vectors. CDMOs have experience with industrial-scale gene therapy workflows and can provide reliable and efficient production processes.
The yield challenge
The largest hurdle in viral vector production is yield. All cell lines have inhibitory sequences that influence vector production. And much remains unknown about the viral capsids themselves. For example, viral capsid proteins may ungergo different post-translational modifications in different manufacturing processes. Some critical structural features may need to be preserved to ensure in vivo potency.
“If yield could be doubled, it would be possible to reduce costs by a factor of two,” says Thomas VanCott, PhD, global head of product development at Catalent’s cell and gene therapy business unit. Catalent was one of the first facilities to produce the AAV vector and the first FDA-licensed CDMO for gene therapy products.
VanCott suggests that developers may be unable to make enough AAV capsids per cell or enough functional capsids. Another difficulty is that the transfection process is complicated and requires optimization. Although to some degree the process is transferrable, each plasmid system is a little different. Plasmid DNA, the core ingredient, is manufactured by just a few companies.
“They do it well but cannot meet demand,” VanCott asserts. “To stay on timeline and to have uniformity throughout the process from research lots through GMP grade, we have invested in bringing that capability in-house. Also, we recently announced the acquisition of Belgium-based Delphi Genetics.”
In downstream processing, the functional capsids must be purified away from the empty ones. Ultracentrifugation works well but is labor intensive and slow. Although different chromatographic techniques perform better, a more efficient approach using continuous flow would be of value in addition to improved analytical methods to measure the percentage of empty versus full capsids.
“Viral vectors must be intact and carry the gene of interest,” VanCott emphasizes. “Our platform minimizes the parameters that need to be modulated to lock in a process quicker. The key gets down to securing the supplies with the visibility of lead time.”
Materials, such as single-use bioreactor bags, media, and other reagents for manufacturing, may be hard to secure. The manufacture of COVID-19 vaccines uses similar supplies and has priority in the supply chain.
Proprietary cell lines
For gene therapy, the delivery workhorses are AAV, adenovirus, and lentivirus. If they are to be produced efficiently, manufacturers need a suitable cell line. “Ideally, a stable cell line should be used to minimize batch-to-batch variation,” says Jeffrey Hung, PhD, chief commercial officer, Vigene Biosciences. “But cell lines are very tricky to produce and maintain because of some inherent toxicity of the viral genes when they integrate into the cell genome.”

Vigene has developed mammalian and insect cell lines for virus manufacturing in either suspension or adherent culture. The company notes that the characteristics of the selected cell line (origin/derivation, doubling time, and permissiveness for viral infection and replication) determine the efficiency of viral productivity, whereas the growth conditions determine the requisite downstream processing methods and release tests for the final drug product.
Because insect cells are smaller, more of them can be packed into a set volume. And they offer higher yield for AAV culture. Vigene’s insect cell line technology uses the baculovirus expression system to produce AAV vectors in Sf9 cells under serum-free conditions. However, this is not a plug-and-play technology. Once the AAV is packaged, the baculovirus must be purified away.
For other cells, such as HeLa cells, removal of any residual cellular DNA must be demonstrated. Each cell line presents challenges.
“There are many opportunities for improvements in cell lines,” notes Hung. For example, cell lines could offer better packaging efficiency and greater stability. They could address the potential harms associated with residual DNA. They could also facilitate gains in manufacturability and scalability.
Vigene Biosciences supports AAV, adenovirus, lentivirus, and retrovirus production. In addition, the company offers services to support “everything from basic research to launching a commercial product.”
“A viral vector is just a means to an end,” Hung adds. “Our customers can be anywhere in the development cycle—at the conceptual stage or at mid-scale or clinical-grade production. Sometimes customers have produced certain preclinical-grade products. These customers will find that it is advantageous for them to keep the same cell platform. This option is available for production continuity.”
Purification and analysis
Process optimization is complex. Upstream scientists must cope with multiple culture and transfection parameters, whereas downstream scientists must cope with the absence of standard platforms that work for all vectors, serotypes, and expression systems. Scientists face some very specific challenges, such as the separation of empty and full AAV capsids, as well as the need to adapt the downstream bioprocess for each serotype.
Complex analytical methods and requirements take time to establish. Processes need to be sufficiently productive in terms of overall efficiency, yield, purity, and characterization to ensure robustness to increase the chance of regulatory success as well as derisk GMP production campaigns.
“We have invested heavily in our high-throughput platforms, such as the Ambr platform, for cell culture, in combination with our design of experiments software,” says Amélie Boulais Raveneau, head of market entry strategy, Viral-Based Therapeutics, Sartorius Stedim Biotech. “This enables manufacturers to take a systematic approach toward optimizing cell culture and transfection parameters.”
The recent acquisition of BIA Separations adds that company’s “monoliths,” monolithic chromatography columns, to the Sartorius portfolio. The monoliths have unique chemistries that allow for high-resolution AAV purification. Sartorius also gains a development laboratory that develops AAV processes.

“We have a platform that can be optimized to rapidly purify all serotypes of AAV and enable efficient empty/full capsid separation,” Boulais Raveneau notes. “The platform does not rely on expensive AAV affinity assays. Instead, it utilizes a combination of orthogonal chemistry to achieve the level of purity and yield required.”
The BIA acquisition also helps Sartorius offer scientists an expanded toolbox of reliable technologies to measure critical quality parameters and performance attributes. For example, BIA’s CIMac analytical chromatography columns are efficient tools for quantification and for getting the full picture of the sample through the fingerprinting method. Earlier, Sartorius acquired Danaher Life Sciences’ FortéBio business, which brought Sartorius the Octet platform, which allows rapid at-line virus titer quantification to serve as an alternative to the time-consuming ELISA assay.
Processes need to be suitable for GMP production. They also need to be standardized. The fulfillment of these needs is being facilitated by the transition to suspension processes and by research into AAV-agnostic purification trains.
New manufacturing platforms
The continued use of adherent cultures, legacy cell lines, and unoptimized unit operations means that developers or their CDMOs need to devote additional time to resolving yield and efficiency issues. Even experienced developers tend to rely on CDMOs to resolve these issues and bring processes to commercial scale. CDMOs, however, have capacity limitations, so there is a bottleneck of available slots for production. Furthermore, not every CDMO is a good match for every developer in terms of process capabilities.
“MilliporeSigma is taking a comprehensive approach to meeting the need for more efficient viral vector production,” says Angela Myers, the company’s head of gene editing and novel modalities. “To address technical challenges, we created the VirusExpress Lentiviral Production Platform, which combines a high-performance packaging cell line with a chemically defined medium and optimized suspension process to enhance the scalability of upstream operations.
“Along with our work to develop templated processes for lentiviral and AAV production, and a forthcoming VirusExpress platform for AAV, we strive to create the new platform standard for viral vector manufacturing. To address industry logistical challenges, we recently invested in a $121 million expansion of our viral vector manufacturing CDMO facilities in Carlsbad, CA. Implementing our product technologies will allow us to manufacture vectors better and faster.”
Coming online at the end of 2021, the new facility will accommodate 1,000-L single-use bioreactors to add both scale and production slots to service the growing need for high-quality viral vectors. The increased recognition of the need to transition to suspension production demonstrates that more of the industry is not only recognizing the challenges of traditional vector production, but also thinking ahead about meeting commercial scale and regulatory needs.
Furthermore, technology developers are creating fit-for-purpose tools to address unit operation challenges unique to viral vectors. There is a strong desire to proactively adopt the lessons learned from the commercialization of other biologics over the past three decades.
Quality evidence of clonality
A single-cell cloning stage is necessary in viral vector production. “Clonality relates to a stage in the manufacturing process where regulatory bodies require quality evidence that the process has been brought down to a single cell to minimize the heterogeneity within the cell bank to limit process variability,” says Duncan Borthwick, PhD, global marketing manager, Solentim. “Our technology is used to assure clonality in the production of cell lines and also to increase the efficiency of cell growth.”
The Solentim technology, which consists of the company’s VIPS (Verified In-Situ Plate Seeding) and Cell Metric instruments, dispenses single cells and captures images to provide quality evidence of clonality. According to the company, the technology is the gold standard for capturing evidence of clonality for regulatory submission. Users include GlaxoSmithKline, Thermo Fisher, and BrammerBio (acquired by Thermo Fisher in 2019).

VIPS dispenses cells at very low pressure in nanoliter droplets into plate wells. Each cell is imaged in the bottom of its dry well as evidence of seeding. After the well is automatically filled with media, the Cell Metric whole-well cell imager takes over and images at day 0 for a double lock of assurance. Daily imaging continues to determine if the division rate logically matches to that of a single cell, providing additional evidence.
Applying the Solentim technology is an attractive alternative to using manual limiting dilution techniques and statistics. With the Solentim technology, it is possible to accumulate the data needed to make a solid case to regulators. “Getting the clonality stage right is massively important for process engineering,” says Borthwick. “Insufficient data would have a massive impact on timelines and regulatory progression.”
Solentim’s InstiGRO growth supplements assist in more rapid cell growth, helping individual cells survive the lonely process and positively impacting the workflow. For instance, GlaxoSmithKline reported an 11-fold increase in clonal outgrowth. InstiTHAW aids the freezing and thawing process.
The post The Next Generation of Cell Factories for Viral Vector Production appeared first on GEN - Genetic Engineering and Biotechnology News.
fda adenovirus preclinical genetic therapy dna covid-19 goldInternational
Acadia’s Nuplazid fails PhIII study due to higher-than-expected placebo effect
After years of trying to expand the market territory for Nuplazid, Acadia Pharmaceuticals might have hit a dead end, with a Phase III fail in schizophrenia…

After years of trying to expand the market territory for Nuplazid, Acadia Pharmaceuticals might have hit a dead end, with a Phase III fail in schizophrenia due to the placebo arm performing better than expected.
 Steve Davis
Steve Davis“We will continue to analyze these data with our scientific advisors, but we do not intend to conduct any further clinical trials with pimavanserin,” CEO Steve Davis said in a Monday press release. Acadia’s stock $ACAD dropped by 17.41% before the market opened Tuesday.
Pimavanserin, a serotonin inverse agonist and also a 5-HT2A receptor antagonist, is already in the market with the brand name Nuplazid for Parkinson’s disease psychosis. Efforts to expand into other indications such as Alzheimer’s-related psychosis and major depression have been unsuccessful, and previous trials in schizophrenia have yielded mixed data at best. Its February presentation does not list other pimavanserin studies in progress.
The Phase III ADVANCE-2 trial investigated 34 mg pimavanserin versus placebo in 454 patients who have negative symptoms of schizophrenia. The study used the negative symptom assessment-16 (NSA-16) total score as a primary endpoint and followed participants up to week 26. Study participants have control of positive symptoms due to antipsychotic therapies.
The company said that the change from baseline in this measure for the treatment arm was similar between the Phase II ADVANCE-1 study and ADVANCE-2 at -11.6 and -11.8, respectively. However, the placebo was higher in ADVANCE-2 at -11.1, when this was -8.5 in ADVANCE-1. The p-value in ADVANCE-2 was 0.4825.
In July last year, another Phase III schizophrenia trial — by Sumitomo and Otsuka — also reported negative results due to what the company noted as Covid-19 induced placebo effect.
According to Mizuho Securities analysts, ADVANCE-2 data were disappointing considering the company applied what it learned from ADVANCE-1, such as recruiting patients outside the US to alleviate a high placebo effect. The Phase III recruited participants in Argentina and Europe.
Analysts at Cowen added that the placebo effect has been a “notorious headwind” in US-based trials, which appears to “now extend” to ex-US studies. But they also noted ADVANCE-1 reported a “modest effect” from the drug anyway.
Nonetheless, pimavanserin’s safety profile in the late-stage study “was consistent with previous clinical trials,” with the drug having an adverse event rate of 30.4% versus 40.3% with placebo, the company said. Back in 2018, even with the FDA approval for Parkinson’s psychosis, there was an intense spotlight on Nuplazid’s safety profile.
Acadia previously aimed to get Nuplazid approved for Alzheimer’s-related psychosis but had many hurdles. The drug faced an adcomm in June 2022 that voted 9-3 noting that the drug is unlikely to be effective in this setting, culminating in a CRL a few months later.
As for the company’s next R&D milestones, Mizuho analysts said it won’t be anytime soon: There is the Phase III study for ACP-101 in Prader-Willi syndrome with data expected late next year and a Phase II trial for ACP-204 in Alzheimer’s disease psychosis with results anticipated in 2026.
Acadia collected $549.2 million in full-year 2023 revenues for Nuplazid, with $143.9 million in the fourth quarter.
depression covid-19 treatment fda clinical trials europeUncategorized
Digital Currency And Gold As Speculative Warnings
Over the last few years, digital currencies and gold have become decent barometers of speculative investor appetite. Such isn’t surprising given the evolution…
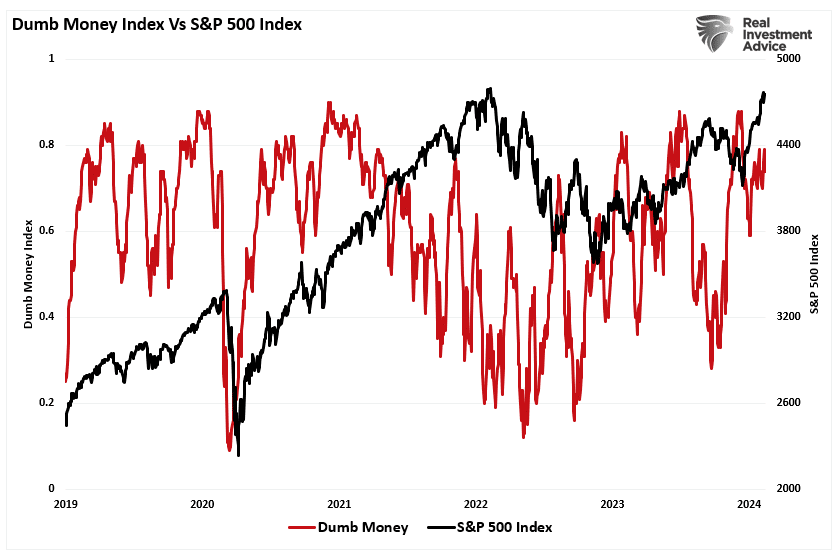
Over the last few years, digital currencies and gold have become decent barometers of speculative investor appetite. Such isn’t surprising given the evolution of the market into a “casino” following the pandemic, where retail traders have increased their speculative appetites.
“Such is unsurprising, given that retail investors often fall victim to the psychological behavior of the “fear of missing out.” The chart below shows the “dumb money index” versus the S&P 500. Once again, retail investors are very long equities relative to the institutional players ascribed to being the “smart money.””
“The difference between “smart” and “dumb money” investors shows that, more often than not, the “dumb money” invests near market tops and sells near market bottoms.”

That enthusiasm has increased sharply since last November as stocks surged in hopes that the Federal Reserve would cut interest rates. As noted by Sentiment Trader:
“Over the past 18 weeks, the straight-up rally has moved us to an interesting juncture in the Sentiment Cycle. For the past few weeks, the S&P 500 has demonstrated a high positive correlation to the ‘Enthusiasm’ part of the cycle and a highly negative correlation to the ‘Panic’ phase.”

That frenzy to chase the markets, driven by the psychological bias of the “fear of missing out,” has permeated the entirety of the market. As noted in “This Is Nuts:”
“Since then, the entire market has surged higher following last week’s earnings report from Nvidia (NVDA). The reason I say “this is nuts” is the assumption that all companies were going to grow earnings and revenue at Nvidia’s rate. There is little doubt about Nvidia’s earnings and revenue growth rates. However, to maintain that growth pace indefinitely, particularly at 32x price-to-sales, means others like AMD and Intel must lose market share.”

Of course, it is not just a speculative frenzy in the markets for stocks, specifically anything related to “artificial intelligence,” but that exuberance has spilled over into gold and cryptocurrencies.
Birds Of A Feather
There are a couple of ways to measure exuberance in the assets. While sentiment measures examine the broad market, technical indicators can reflect exuberance on individual asset levels. However, before we get to our charts, we need a brief explanation of statistics, specifically, standard deviation.
As I discussed in “Revisiting Bob Farrell’s 10 Investing Rules”:
“Like a rubber band that has been stretched too far – it must be relaxed in order to be stretched again. This is exactly the same for stock prices that are anchored to their moving averages. Trends that get overextended in one direction, or another, always return to their long-term average. Even during a strong uptrend or strong downtrend, prices often move back (revert) to a long-term moving average.”
The idea of “stretching the rubber band” can be measured in several ways, but I will limit our discussion this week to Standard Deviation and measuring deviation with “Bollinger Bands.”
“Standard Deviation” is defined as:
“A measure of the dispersion of a set of data from its mean. The more spread apart the data, the higher the deviation. Standard deviation is calculated as the square root of the variance.”
In plain English, this means that the further away from the average that an event occurs, the more unlikely it becomes. As shown below, out of 1000 occurrences, only three will fall outside the area of 3 standard deviations. 95.4% of the time, events will occur within two standard deviations.

A second measure of “exuberance” is “relative strength.”
“In technical analysis, the relative strength index (RSI) is a momentum indicator that measures the magnitude of recent price changes to evaluate overbought or oversold conditions in the price of a stock or other asset. The RSI is displayed as an oscillator (a line graph that moves between two extremes) and can read from 0 to 100.
Traditional interpretation and usage of the RSI are that values of 70 or above indicate that a security is becoming overbought or overvalued and may be primed for a trend reversal or corrective pullback in price. An RSI reading of 30 or below indicates an oversold or undervalued condition.” – Investopedia
With those two measures, let’s look at Nvidia (NVDA), the poster child of speculative momentum trading in the markets. Nvidia trades more than 3 standard deviations above its moving average, and its RSI is 81. The last time this occurred was in July of 2023 when Nvidia consolidated and corrected prices through November.

Interestingly, gold also trades well into 3 standard deviation territory with an RSI reading of 75. Given that gold is supposed to be a “safe haven” or “risk off” asset, it is instead getting swept up in the current market exuberance.

The same is seen with digital currencies. Given the recent approval of spot, Bitcoin exchange-traded funds (ETFs), the panic bid to buy Bitcoin has pushed the price well into 3 standard deviation territory with an RSI of 73.

In other words, the stock market frenzy to “buy anything that is going up” has spread from just a handful of stocks related to artificial intelligence to gold and digital currencies.
It’s All Relative
We can see the correlation between stock market exuberance and gold and digital currency, which has risen since 2015 but accelerated following the post-pandemic, stimulus-fueled market frenzy. Since the market, gold and cryptocurrencies, or Bitcoin for our purposes, have disparate prices, we have rebased the performance to 100 in 2015.
Gold was supposed to be an inflation hedge. Yet, in 2022, gold prices fell as the market declined and inflation surged to 9%. However, as inflation has fallen and the stock market surged, so has gold. Notably, since 2015, gold and the market have moved in a more correlated pattern, which has reduced the hedging effect of gold in portfolios. In other words, during the subsequent market decline, gold will likely track stocks lower, failing to provide its “wealth preservation” status for investors.

The same goes for cryptocurrencies. Bitcoin is substantially more volatile than gold and tends to ebb and flow with the overall market. As sentiment surges in the S&P 500, Bitcoin and other cryptocurrencies follow suit as speculative appetites increase. Unfortunately, for individuals once again piling into Bitcoin to chase rising prices, if, or when, the market corrects, the decline in cryptocurrencies will likely substantially outpace the decline in market-based equities. This is particularly the case as Wall Street can now short the spot-Bitcoin ETFs, creating additional selling pressure on Bitcoin.

Just for added measure, here is Bitcoin versus gold.

Not A Recommendation
There are many narratives surrounding the markets, digital currency, and gold. However, in today’s market, more than in previous years, all assets are getting swept up into the investor-feeding frenzy.
Sure, this time could be different. I am only making an observation and not an investment recommendation.
However, from a portfolio management perspective, it will likely pay to remain attentive to the correlated risk between asset classes. If some event causes a reversal in bullish exuberance, cash and bonds may be the only place to hide.
The post Digital Currency And Gold As Speculative Warnings appeared first on RIA.
bonds pandemic sp 500 equities stocks bitcoin currencies goldInternational
Four Years Ago This Week, Freedom Was Torched
Four Years Ago This Week, Freedom Was Torched
Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare…

Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare quotes the soothsayer’s warning Julius Caesar about what turned out to be an impending assassination on March 15. The death of American liberty happened around the same time four years ago, when the orders went out from all levels of government to close all indoor and outdoor venues where people gather.
It was not quite a law and it was never voted on by anyone. Seemingly out of nowhere, people who the public had largely ignored, the public health bureaucrats, all united to tell the executives in charge – mayors, governors, and the president – that the only way to deal with a respiratory virus was to scrap freedom and the Bill of Rights.
And they did, not only in the US but all over the world.
The forced closures in the US began on March 6 when the mayor of Austin, Texas, announced the shutdown of the technology and arts festival South by Southwest. Hundreds of thousands of contracts, of attendees and vendors, were instantly scrapped. The mayor said he was acting on the advice of his health experts and they in turn pointed to the CDC, which in turn pointed to the World Health Organization, which in turn pointed to member states and so on.
There was no record of Covid in Austin, Texas, that day but they were sure they were doing their part to stop the spread. It was the first deployment of the “Zero Covid” strategy that became, for a time, official US policy, just as in China.
It was never clear precisely who to blame or who would take responsibility, legal or otherwise.
This Friday evening press conference in Austin was just the beginning. By the next Thursday evening, the lockdown mania reached a full crescendo. Donald Trump went on nationwide television to announce that everything was under control but that he was stopping all travel in and out of US borders, from Europe, the UK, Australia, and New Zealand. American citizens would need to return by Monday or be stuck.
Americans abroad panicked while spending on tickets home and crowded into international airports with waits up to 8 hours standing shoulder to shoulder. It was the first clear sign: there would be no consistency in the deployment of these edicts.
There is no historical record of any American president ever issuing global travel restrictions like this without a declaration of war. Until then, and since the age of travel began, every American had taken it for granted that he could buy a ticket and board a plane. That was no longer possible. Very quickly it became even difficult to travel state to state, as most states eventually implemented a two-week quarantine rule.
The next day, Friday March 13, Broadway closed and New York City began to empty out as any residents who could went to summer homes or out of state.
On that day, the Trump administration declared the national emergency by invoking the Stafford Act which triggers new powers and resources to the Federal Emergency Management Administration.
In addition, the Department of Health and Human Services issued a classified document, only to be released to the public months later. The document initiated the lockdowns. It still does not exist on any government website.
The White House Coronavirus Response Task Force, led by the Vice President, will coordinate a whole-of-government approach, including governors, state and local officials, and members of Congress, to develop the best options for the safety, well-being, and health of the American people. HHS is the LFA [Lead Federal Agency] for coordinating the federal response to COVID-19.
Closures were guaranteed:
Recommend significantly limiting public gatherings and cancellation of almost all sporting events, performances, and public and private meetings that cannot be convened by phone. Consider school closures. Issue widespread ‘stay at home’ directives for public and private organizations, with nearly 100% telework for some, although critical public services and infrastructure may need to retain skeleton crews. Law enforcement could shift to focus more on crime prevention, as routine monitoring of storefronts could be important.
In this vision of turnkey totalitarian control of society, the vaccine was pre-approved: “Partner with pharmaceutical industry to produce anti-virals and vaccine.”
The National Security Council was put in charge of policy making. The CDC was just the marketing operation. That’s why it felt like martial law. Without using those words, that’s what was being declared. It even urged information management, with censorship strongly implied.
The timing here is fascinating. This document came out on a Friday. But according to every autobiographical account – from Mike Pence and Scott Gottlieb to Deborah Birx and Jared Kushner – the gathered team did not meet with Trump himself until the weekend of the 14th and 15th, Saturday and Sunday.
According to their account, this was his first real encounter with the urge that he lock down the whole country. He reluctantly agreed to 15 days to flatten the curve. He announced this on Monday the 16th with the famous line: “All public and private venues where people gather should be closed.”
This makes no sense. The decision had already been made and all enabling documents were already in circulation.
There are only two possibilities.
One: the Department of Homeland Security issued this March 13 HHS document without Trump’s knowledge or authority. That seems unlikely.
Two: Kushner, Birx, Pence, and Gottlieb are lying. They decided on a story and they are sticking to it.
Trump himself has never explained the timeline or precisely when he decided to greenlight the lockdowns. To this day, he avoids the issue beyond his constant claim that he doesn’t get enough credit for his handling of the pandemic.
With Nixon, the famous question was always what did he know and when did he know it? When it comes to Trump and insofar as concerns Covid lockdowns – unlike the fake allegations of collusion with Russia – we have no investigations. To this day, no one in the corporate media seems even slightly interested in why, how, or when human rights got abolished by bureaucratic edict.
As part of the lockdowns, the Cybersecurity and Infrastructure Security Agency, which was and is part of the Department of Homeland Security, as set up in 2018, broke the entire American labor force into essential and nonessential.
They also set up and enforced censorship protocols, which is why it seemed like so few objected. In addition, CISA was tasked with overseeing mail-in ballots.
Only 8 days into the 15, Trump announced that he wanted to open the country by Easter, which was on April 12. His announcement on March 24 was treated as outrageous and irresponsible by the national press but keep in mind: Easter would already take us beyond the initial two-week lockdown. What seemed to be an opening was an extension of closing.
This announcement by Trump encouraged Birx and Fauci to ask for an additional 30 days of lockdown, which Trump granted. Even on April 23, Trump told Georgia and Florida, which had made noises about reopening, that “It’s too soon.” He publicly fought with the governor of Georgia, who was first to open his state.
Before the 15 days was over, Congress passed and the president signed the 880-page CARES Act, which authorized the distribution of $2 trillion to states, businesses, and individuals, thus guaranteeing that lockdowns would continue for the duration.
There was never a stated exit plan beyond Birx’s public statements that she wanted zero cases of Covid in the country. That was never going to happen. It is very likely that the virus had already been circulating in the US and Canada from October 2019. A famous seroprevalence study by Jay Bhattacharya came out in May 2020 discerning that infections and immunity were already widespread in the California county they examined.
What that implied was two crucial points: there was zero hope for the Zero Covid mission and this pandemic would end as they all did, through endemicity via exposure, not from a vaccine as such. That was certainly not the message that was being broadcast from Washington. The growing sense at the time was that we all had to sit tight and just wait for the inoculation on which pharmaceutical companies were working.
By summer 2020, you recall what happened. A restless generation of kids fed up with this stay-at-home nonsense seized on the opportunity to protest racial injustice in the killing of George Floyd. Public health officials approved of these gatherings – unlike protests against lockdowns – on grounds that racism was a virus even more serious than Covid. Some of these protests got out of hand and became violent and destructive.
Meanwhile, substance abuse rage – the liquor and weed stores never closed – and immune systems were being degraded by lack of normal exposure, exactly as the Bakersfield doctors had predicted. Millions of small businesses had closed. The learning losses from school closures were mounting, as it turned out that Zoom school was near worthless.
It was about this time that Trump seemed to figure out – thanks to the wise council of Dr. Scott Atlas – that he had been played and started urging states to reopen. But it was strange: he seemed to be less in the position of being a president in charge and more of a public pundit, Tweeting out his wishes until his account was banned. He was unable to put the worms back in the can that he had approved opening.
By that time, and by all accounts, Trump was convinced that the whole effort was a mistake, that he had been trolled into wrecking the country he promised to make great. It was too late. Mail-in ballots had been widely approved, the country was in shambles, the media and public health bureaucrats were ruling the airwaves, and his final months of the campaign failed even to come to grips with the reality on the ground.
At the time, many people had predicted that once Biden took office and the vaccine was released, Covid would be declared to have been beaten. But that didn’t happen and mainly for one reason: resistance to the vaccine was more intense than anyone had predicted. The Biden administration attempted to impose mandates on the entire US workforce. Thanks to a Supreme Court ruling, that effort was thwarted but not before HR departments around the country had already implemented them.
As the months rolled on – and four major cities closed all public accommodations to the unvaccinated, who were being demonized for prolonging the pandemic – it became clear that the vaccine could not and would not stop infection or transmission, which means that this shot could not be classified as a public health benefit. Even as a private benefit, the evidence was mixed. Any protection it provided was short-lived and reports of vaccine injury began to mount. Even now, we cannot gain full clarity on the scale of the problem because essential data and documentation remains classified.
After four years, we find ourselves in a strange position. We still do not know precisely what unfolded in mid-March 2020: who made what decisions, when, and why. There has been no serious attempt at any high level to provide a clear accounting much less assign blame.
Not even Tucker Carlson, who reportedly played a crucial role in getting Trump to panic over the virus, will tell us the source of his own information or what his source told him. There have been a series of valuable hearings in the House and Senate but they have received little to no press attention, and none have focus on the lockdown orders themselves.
The prevailing attitude in public life is just to forget the whole thing. And yet we live now in a country very different from the one we inhabited five years ago. Our media is captured. Social media is widely censored in violation of the First Amendment, a problem being taken up by the Supreme Court this month with no certainty of the outcome. The administrative state that seized control has not given up power. Crime has been normalized. Art and music institutions are on the rocks. Public trust in all official institutions is at rock bottom. We don’t even know if we can trust the elections anymore.
In the early days of lockdown, Henry Kissinger warned that if the mitigation plan does not go well, the world will find itself set “on fire.” He died in 2023. Meanwhile, the world is indeed on fire. The essential struggle in every country on earth today concerns the battle between the authority and power of permanent administration apparatus of the state – the very one that took total control in lockdowns – and the enlightenment ideal of a government that is responsible to the will of the people and the moral demand for freedom and rights.
How this struggle turns out is the essential story of our times.
CODA: I’m embedding a copy of PanCAP Adapted, as annotated by Debbie Lerman. You might need to download the whole thing to see the annotations. If you can help with research, please do.
* * *
Jeffrey Tucker is the author of the excellent new book 'Life After Lock-Down'
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International4 days ago
International4 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges






















