International
Sanofi 2022: Facing forward
Following its Play to Win strategy, Sanofi looks to the future with a new corporate identity and charitable foundations.

Following its Play to Win strategy, Sanofi looks to the future with a new corporate identity and charitable foundations.
By Christiane Truelove • chris.truelove@medadnews.com
Sanofi
54, rue La Boétie
75008 Paris, France
+ 33 (0)1 53 77 40 00 • sanofi.com
| Financial Performance | ||||
| 2021 | 2020 | 1H 2022 | 1H 2021 | |
| Revenue | $44,671 | $42,637 | $23,412 | $20,507 |
| Net income | $7,362 | $14,544 | $5,435 | $4,434 |
| Diluted EPS | $5.86 | $11.55 | $4.35 | $3.55 |
| R&D expense | $6,734 | $6,542 | $3,723 | $3,150 |
| All figures except EPS are in millions of dollars and were translated using the Federal Reserve Board’s aver- age rate of exchange in 2021: €1.183. | ||||
Best-selling products
All sales are in millions of dollars and were translated using theFederal Reserve Board’s average rate of exchange in 2021: €1.183.
2021 sales
- Dupixent $6,210
- Lantus $2,950
- Aubagio $2,313
- Lovenox $1,758
- Myozyme, Lumizyme $1,187
- Toujeo $1,146
- Plavix $1,099 $
- Fabrazyme $998
- Cerezyme $808
- Eloctate $666
- Jevtana $538
1H 2022
- Dupixent $4,232
- Lantus $1,504
- Aubagio $1,203
- Lovenox $845
- Toujeo $640
- Plavix $601
- Myozyme, Lumizyme $576
- Fabrazyme $542
- Cerezyme $434
- Eloctate $344
- Avapro/Aprovel, CoAprovel $289
- Alprolix $280
Outcomes Creativity Index Score: 22
- Manny Awards — N/A
- Cannes Lions — N/A
- Clio Health — N/A
- Creative Floor Awards — 19
- London International Awards – N/A
- MM+M Awards — 3
- One Show — N/A
In summing up Sanofi’s performance at the end of 2021, CEO Paul Hudson cites “impressive record sales” of Dupixent, and “another year of record influenza sales in the company’s vaccines division.”
“In R&D, we continue to be relentless in our commitment to expand our innovative pipeline,” Hudson stated. “Last quarter (Q4 2021), Sanofi achieved a new milestone, a first in recent years, by moving seven molecules into Phase I and seven pipeline programs into Phase II trials, showcasing our success in rapidly advancing potentially transformative medicines. We further strengthened our R&D capabilities with a series of value-creating M&A transactions in 2021. “
Hudson continued, “Our excellent financial performance validates our ability to increase profitability through improved product mix, supported by expense management and the reinvestment of savings behind our growth drivers, all of which puts us on a trajectory to achieving our 2022 financial targets.”
And in their strategy, Sanofi executives are thinking about much more than just financial results. In February 2022, Sanofi kicked unveiled a new “bold and unifying” corporate brand that “supports the modernization and transformation the company launched in December 2019.”
“Over the last 50 years, Sanofi has grown into a diverse, global healthcare leader, with a rich heritage of patient-centric scientific discovery,” company executives say. “This history includes the first treatments for many rare diseases and the establishment of standards of care in diabetes and cardiovascular disease. Sanofi’s commitment to public health has helped protect hundreds of millions of people from influenza every year for decades and pushed polio to the brink of eradication, while its scientific vision has led to breakthrough innovations in the treatment of inflammatory diseases.”
Management says with its roots in a variety of diverse companies, Sanofi is now the combination of many cultures, identities and brands,. The new brand brings this diverse history together in a single common identity for the first time, manifesting the company’s journey and highlighting an ambitious strategy for the future.
“As we approach the half-century mark of our company’s existence, we have undertaken the most important transformation and modernization in our history,” Hudson says. “In 2019, we launched our ‘Play to Win’ strategy, which focuses on applying our platform for innovation to produce first- and best-in-class treatments and vaccines. Our new brand is a natural and important next step in this journey and represents the integrated way in which the company will work to achieve our shared ambition to transform the practice of medicine.”
Under the new corporate identity, the vaccine unit Sanofi Pasteur and the specialty medicines unit Sanofi Genzyme, as well as all other acquired brands, will be united under the singular Sanofi name and brand.
“These brands have for years symbolized the impact that innovation can have on people’s lives,” company executives say. “Thinking, acting, and behaving as a single entity under a new shared purpose and identity will position Sanofi to have greater impact by more strategically applying the resources that exist across the company to drive innovations that matter.”
Management maintains that the new logo (see this magazine’s cover) is a representation of Sanofi’s new purpose and ambition, which is inspired by the simple and motion-oriented codes of the tech industry. The two purple dots embody the scientific journey between a starting point – the curiosity of questioning the status-quo and wondering “what if?” – and a finish line – the eureka moment where innovative solutions are unlocked to impact people’s lives.
“With our new brand, we have sought to provide our people, our partners, patients and healthcare professionals with a clear and strong understanding of who we are and what we are set to achieve,” says Josep Catllà, head of corporate affairs at Sanofi. “Sanofi’s attitude is humble, authentic – and a little bit unconventional, too. We believe that our new brand and logo carve out a unique space in the healthcare industry that perfectly represents our new purpose to chase the miracles of science to improve people’s lives.”
In another effort to display its vision beyond just pharmaceuticals, Sanofi in May 2022 launched Foundation S – The Sanofi Collective, its philanthropic endowment fund aiming to create healthier futures for generations. Using donations, partnerships and collective action, Foundation S will focus on three critical areas: childhood cancer, the health of communities most vulnerable to the effects of climate change and pollution, and access to lifesaving medicines and vaccines.
Foundation S will incorporate as its cornerstone initiative the former Sanofi Espoir Foundation’s “My Child Matters” program in childhood cancer. Additionally, Foundation S will fund awareness and research for childhood cancer in various countries.
Foundation S also will work to increase the health resilience of vulnerable populations most impacted by climate change and pollution, collaborating with the international NGO Friendship, with a project focused on Bangladesh’s hard-to-reach, climate-vulnerable islands of Gaibandha. There, Foundation S will help train healthcare workers, and fund satellite clinics and a floating hospital in a region outside mainstream healthcare services.
Also, Foundation S will leverage Sanofi’s longstanding emergency aid expertise and improve the connection between humanitarian and development financing, renewing Sanofi’s emergency aid donations program and expanding proactive support notably for displaced populations.
“The launch of Foundation S is a new cornerstone of Sanofi’s commitment to society and a pivotal moment for the life of our company and our people,” Hudson says. “With Foundation S, we aim to weigh in and act on targeted areas where we know we can make a real difference for populations exposed to health difficulties.”
Advancing the company’s fully integrated social impact strategy once more in July 2022, Sanofi launched Impact, a dedicated brand for non-profit distribution to enable the secure distribution of 30 Sanofi medicines in 40 lower-income countries.
The Impact brand includes insulin, glibenclamide, and oxaliplatin among others. Considered essential by the World Health Organization, these medicines cover a wide range of therapeutic areas, including diabetes, cardiovascular disease, tuberculosis, malaria, and cancer.
The launch of the Impact brand is among the steps taken since the formation last year of Sanofi Global Health, a nonprofit unit within the company aiming to increase access to healthcare through the distribution of medicines, and the building and bolstering of local healthcare systems in countries with among the lowest per-capita GDP. Executives say Sanofi Global Health is the first and only global initiative to provide access to such a broad portfolio of medicines in so many countries and across multiple therapeutic areas while funding local support programs and strengthening local inclusive businesses.
“At Sanofi, we believe we have a responsibility to make a difference for the health of those most in need, and we know we have the ability and the ambition to bring about lasting change,” Hudson says. “With critical medicines, relentless drive and impactful partnerships, we can take our innovation beyond the lab and use it to strengthen health systems and access to medicines for those most vulnerable communities of patients. Sanofi Global Health aims to improve the lives of millions of people who now cannot get the help they need. Sanofi’s renewed purpose is to chase the miracles of science to improve people’s lives. And our quest to make life better for all people must include helping to provide better access to care and quality medicines for underserved populations.”
Performance & Outlook
Sanofi’s sales in 2021 grew to €37.76 billion ($44.67 billion), 4.8 percent more than in 2020. First-half 2022 revenue was €19.79 billion ($23.41 billion), 14.2 percent more than in the same period last year.
The company’s 2021 net income was €6.22 billion ($7.36 billion) compared with 2020’s €12.29 billion ($14.54 billion). Diluted earnings per share in 2021 were €4.95 ($5.86) compared with 2020’s €9.76 ($11.55). 2020’s net income and earnings per share reflect the divestment of the Animal Health business that year.
For the first half of 2022, net income was €4.59 billion ($5.43 billion), 22.6 percent more than in the same period last year. Earnings per share were €3.68 ($4.35), 22.7 percent more than in first-half 2021.
“As we continue to deliver ahead of schedule on our Play to Win strategy, we are confident in our business outlook for the second half and as a result, we are reiterating our commitment to achieving the BOI margin target of 30 percent in 2022,” Hudson says.
Sanofi’s top pharma product in terms of 2021 sales was Dupixent, which generated €5.25
billion ($6.21 billion) compared with €3.53 billion ($4.18 billion) in 2020.
First-half 2022 Dupixent sales were €3.58 billion ($4.23 billion), 44.4 percent more than in the first six months of 2021.
In a distant second place was the insulin Lantus, generating €2.49 billion ($2.95 billion), 6.3 percent less than in 2020. Sales in the first half of 2022 continued to decline compared with the same period last year, dropping to €1.27 billion ($1.5 billion), 6.7 percent less than in first-half 2021.
Coming in at No. 3 was the multiple sclerosis drug Aubagio, at €1.96 billion ($2.31 billion), a decline of 4.4 percent from 2021. In first-half 2022, Aubagio sales totaled €1.27 billion ($1.5 billion), another 4.4 percent less than in the first six months of 2021.

Lovenox sales increased to $1.76 billion in 2021, but decreased to $845 million for the first six months of 2022.
Sanofi’s fourth best-selling product in 2021 was the antithrombotic Lovenox, at €1.49 billion ($1.76 billion). The drug’s performance, despite competition from biosimilars, was driven by sales growth in what Sanofi designates as the Rest of the World region and the effect of WHO recommendations on the use of low molecular weight heparins in hospitalized COVID-19 patients. First-half 2022 sales were €714 million ($845 million).
The company’s fifth best-selling pharma product line in 2021 was the Pompe disease franchise comprising Myozyme and Lumizyme. Together, the products generated €1 billion ($1.19 billion), an increase of 5.8 percent from 2020. First-half 2022 sales were €487 million ($576 million), a 3.3 percent decrease because of the impact of the launch of Sanofi’s Nexviazyme and conversion in the United States and Japan.
No. 6 in 2021 sales was the insulin Toujeo, generating €969 million ($1.15 billion), 6.4 percent more than in the previous year. Sales for first-half 2022 were €541 million ($640 million), 8.2 percent more than in the first six months of 2021.
The seventh best seller in 2021 was the blood thinner Plavix, which generated €929 million ($1.1 billion), 1.8 percent more than in 2020. First-half 2022 sales were €508 million ($601 million), an increase of 4.7 percent compared with first-half 2021.
The Fabry disease drug Fabrazyme was Sanofi’s No. 8 best-selling drug in 2021, with €844 million ($998 million), an increase of 3.3 percent from the previous year. Sales during the first six months of 2022 reached €458 million ($542 million), 11.2 percent more than in the same period of 2021.
The Type 1 Gaucher disease drug Cerezyme had 2021 sales of €683 million ($808 million), about 1 percent less than in 2020. First-half 2022 sales amounted to €367 million ($434 million), 7 percent more compared to the same period last year.
Sanofi’s hemophilia A drug Eloctate posted 2021 sales of €563 million ($666 million), 11.8 percent less than in 2020 due to a decrease in sales in the Rest of World region. For the first six months of 2022, sales totaled €291 million ($344 million), 4.7 percent more than in the same period last year.
Jevtana, for treating prostate cancer, generated 2021 sales of €455 million ($538 million), 15.1 percent less than in 2020. First-half 2022 sales were €203 million ($240 million), down 15.4 percent versus first-half 2021. Decreased sales are attributed to increased generic competition in the United States and Europe.
Pipeline progress

Dupixent indications expanded in 2022 to include eosinophilic esophagitis, with FDA approval making it the first drug in the United States to treat this condition.
Sanofi achieved various pipeline goals throughout 2021. The blockbuster brand Dupixent won FDA approval for treating moderate-to-severe asthma for children aged 6 to 11 years. Dupixent also had positive pivotal trial results in prurigo nodularis and eosinophilic esophagitis.
The company reported that Sanofi had moved various projects into its early-stage pipeline, with seven entering Phase I and seven added to Phase II.
“In 2018, we reimagined innovation in Sanofi R&D and changed the allocation of our resources to focus on delivering transformative medicines for the most urgent unmet patient needs,” stated John Reed, executive VP and global head of R&D. “Since then, we’ve gone through rapid transformation not only of our pipeline, but also our processes so that we can accelerate the pace of delivery for patients. The global pandemic stress-tested our revamped and reinvigorated approaches, pushing our teams to adopt more agile ways of working that are now becoming embedded as the new normal. One of the most remarkable transformations over the past couple of years has been in our immunology and inflammation pipeline, which has become one of the most vibrant elements of the company’s portfolio, with our flagship medicine growing rapidly in numbers of patients treated.”
Reed added, “Immunoscience is the bedrock of our work on immunological and inflammatory diseases, and is driving our progress across multiple therapeutic areas, including neurology, hematology, oncology and, of course, vaccines.”
There have been several notable pipeline achievements so far for 2022. In September, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion for Beyfortus (nirsevimab) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants during their first RSV season. If approved, Beyfortus would be the first and only single-dose passive immunization for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions. Beyfortus is being developed jointly by Sanofi and AstraZeneca.
CHMP also in September recommended the approval of Enjaymo (sutimlimab), a C1 protein (C1s) inhibitor for treatment of hemolytic anemia in adult patients with cold agglutinin disease (CAD). CAD is a rare, serious, and chronic autoimmune hemolytic anemia. Sanofi expects a decision by the end of 2022. The drug was approved by the FDA in February 2022 as the first treatment indicated to decrease the need for red blood cell transfusion due to hemolysis in adults with CAD.
Also in September 2022, positive Phase III data was published in The Lancet for Dupixent in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. The FDA approved the drug in June 2022 for this indication based on the Phase III data. Data from this trial showed that adding Dupixent to low-potency topical corticosteroids (TCS) significantly improved skin clearance and reduced overall disease severity and itch compared to TCS alone (placebo) at 16 weeks.
The drug’s indications expanded again in May 2022, when FDA approved Dupixent for adults and children aged 12 and older with eosinophilic esophagitis (EoE). With this approval, Dupixent became the first medicine specifically indicated to treat EoE in the United States. A regulatory filing for EoE is under review by the European Medicines Agency, and submissions to regulatory authorities in additional countries were planned by the end of 2022.
In September, FDA granted Dupixent approval for prurigo nodularis, making it the only drug approved for this indication.
In August, FDA approved Xenpozyme (olipudase alfa-rpcp) for treating non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD) in adult and pediatric patients. Xenpozyme is the first therapy indicated specifically for the treatment of ASMD, and is the only approved treatment for this disease.
Xenpozyme is an enzyme replacement therapy designed to replace deficient or defective acid sphingomyelinase (ASM), an enzyme that allows for the breakdown of the lipid sphingomyelin. In individuals with ASMD, the insufficient amount of the ASM enzyme means sphingomyelin is poorly metabolized, potentially leading to lifelong accumulation in, and damage to, multiple organs.
“Sanofi teams have been dedicated to bringing hope to patients living with ASMD and their families,” says Bill Sibold, VP, head, specialty care, at Sanofi. “This is a devastating and extremely rare disease that affects both children and adults. The approval of Xenpozyme represents the culmination of bold work done in research and development, and our unwavering commitment to this historically overlooked community.” There are fewer than 120 patients diagnosed with ASMD in the United States, with approximately two-thirds of these patients being children.
Xenpozyme was approved by the European Commission in June 2022. The European Medicines Agency (EMA) granted Xenpozyme PRIority MEdicines (PRIME) designation.
Also in August, FDA accepted for priority review the Biologics License Application (BLA) for efanesoctocog alfa (BIVV001) for the treatment of hemophilia A. The target action date is Feb. 28, 2023. The BLA is supported by data from the XTEND-1 Phase III study. Sanofi executives say the data demonstrate a clinically meaningful prevention of bleeds and superiority to prior factor prophylaxis based on an intra-patient comparison.
Not everything has been a success. Sanofi announced in August that the company is discontinuing the global clinical development program of amcenestrant, an investigational oral selective estrogen receptor degrader (SERD). The decision is based on the outcome of a prespecified interim analysis of the Phase III AMEERA-5 trial evaluating amcenestrant in combination with palbociclib compared with letrozole in combination with palbociclib in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2) advanced breast cancer. An independent data monitoring committee found that amcenestrant in combination with palbociclib did not meet the prespecified boundary for continuation in comparison with the control arm and recommended stopping the trial.
Nexviadyme (avalglucosidase alfa) was approved by the European Commission in June 2022 as a potential new standard of care for the treatment of Pompe disease. The drug is indicated for the treatment of the full spectrum of both late-onset Pompe disease and infantile-onset Pompe disease, and is the first new treatment option approved for the Pompe community in Europe in more than 15 years.
Nexviadyme has also been approved in the United States, Japan, Canada, Switzerland, Australia, Brazil, Taiwan, and the United Arab Emirates. Outside of Europe, the treatment is marketed under the brand name Nexviazyme.
In the area of COVID-19 prevention, Sanofi and GSK in June announced positive data from their vaccine trial, which evaluated an adjuvanted bivalent D614 and Beta (B.1.351) vaccine candidate. Company executives state that the Sanofi-GSK’s vaccine is the first candidate to demonstrate efficacy in a placebo-controlled trial in an environment of high Omicron variant circulation.
Earlier in June, Sanofi reported positive data from two trials conducted with the company’s new next-generation COVID-19 booster vaccine candidate modeled on the Beta variant antigen and including GSK’s pandemic adjuvant. The data supporting this next-generation booster vaccine will be submitted to regulatory authorities and indicate the potential of Sanofi-GSK’s next-generation Beta-based booster to be a relevant response to public health needs.
“With the immunogenicity data from our Beta-booster vaccine, they support our belief that, in a largely seropositive world, a next-generation Beta booster vaccine could provide protection against variants like Omicron,” says Thomas Triomphe, executive VP, vaccines, Sanofi. “mRNA has proven speed to market; we are demonstrating here the efficacy that our recombinant protein platform can provide to the world.
Digital Accelerator
In 2022, Sanofi announced the launch of its first Digital Accelerator to foster its ambition to become a leading digital healthcare company. The Accelerator will develop products and solutions that will support Sanofi’s mission to transform the practice of medicine with the use of digital, data and artificial intelligence (AI).
Management says the Digital Accelerator is first focusing on addressing unmet needs in patients suffering from atopic dermatitis in France, Italy, and Spain. The team is developing an integrated platform and data solution to better engage with healthcare professionals and enhance their awareness as well as their patients’ awareness of the disease and the available treatment options.
“Sanofi’s digital transformation is driven by a business and cultural shift as much as it is by technology,” says Arnaud Robert, executive VP and chief digital officer, Sanofi. “The Digital Accelerator will help us democratize the use of data, develop an agile mindset across the company, and accelerate innovation for patients and healthcare professionals – at speed and scale. Our investment in the Digital Accelerator is another demonstration of our commitment to transform the practice of medicine and deliver better outcomes for patients.”
Sanofi executives say the ongoing digital transformation has already led to significant achievements including accelerating the discovery of new targets using AI; accelerating R&D image analysis using AI, improving clinical trial efficiency by using real-world evidence to reduce the number of patients that must be enrolled and by enabling participants to provide their data and digital biomarkers remotely; and optimizing advertising and promotional spend across multiple markets and products.
By 2025, management predicts that Sanofi’s digital healthcare platform will support new digital businesses, fuel new digital experiences for patients and HCPs, and drive innovation and efficiencies across the entire value chain.
Acquisition/partnerships
Sanofi enhanced itself with two acquisitions in 2021. In December 2021, Sanofi agreed to acquire Amunix, an immuno-oncology company that adds a pipeline with conditionally activated biologics. The company also agreed in December 2021 to acquire Origimm, a biotechnology company specialized in research of skin diseases.
The Amunix acquisition, which was completed during February 2022, brought the Pro-XTEN, XPAT, and XPAC technology to deliver next-generation conditionally activated biologics. Company management says the technology platform is highly complementary to Sanofi’s existing R&D platforms and supports the company’s efforts to accelerate and expand its contributions to innovative medicines for oncology patients. Amunix’s pipeline includes lead candidate AMX-818, a masked HER2-directed TCE that company executives say offers a strong strategic fit with Sanofi’s focus on developing potentially transformative cancer therapies in immuno-oncology.
Origimm adds ORI-001 to Sanofi’s early-stage pipeline. ORI-001 is a therapeutic vaccine candidate for acne vulgaris based on recombinant proteins, which entered preliminary clinical studies in third-quarter 2021. In parallel, Sanofi is working to develop additional antigen versions and expects to leverage its next-generation mRNA platform in a comprehensive Phase I/II trial to start in 2023.
The company continues to explore new partnerships as well. Sanofi and Innovent Biologics announced in August 2022 a collaboration to bring innovative medicines to patients in China with difficult-to-treat cancers. Both companies are committed to accelerating the development and commercialization of two Sanofi key clinical stage oncology assets: Phase III SAR408701 (tusamitamab ravtansine; anti-CEACAM5 antibody-drug conjugate) and Phase II SAR444245 (non-alpha IL-2), combining with sintilimab, the leading checkpoint inhibitor in China.
In addition to the collaboration and license agreement, Sanofi will invest €300 million in Innovent through subscription of new common shares.
“This strategic collaboration with Innovent will not only accelerate the development, market access and future commercialization of two of our key oncology medicines in selected combinations with sintilimab, but also bolster our overall presence in oncology in China,” Reed says. “We look forward to a successful partnership with Innovent, one of the most innovative companies in China, and to leveraging their development capabilities and market leadership in the country.
federal reserve pandemic covid-19 vaccine treatment fda therapy gdp brazil japan canada european europe france spain italy china world health organizationInternational
MIPIM 2024 Reflects Mixed Feelings on CRE Recovery
Reportedly, concerns at the forefront include the plunging commercial real estate market (CRE). During the pandemic, many offices were vacated by staff,…

Reportedly, concerns at the forefront include the plunging commercial real estate market (CRE). During the pandemic, many offices were vacated by staff, and businesses established remote work practices. Since then, the market never fully recovered.
Based on Reuters information, the 20,000 attendees include property giants such as LaSalle, Greystar, AEW, Patrizia and Federated Hermes (FHI.N). Some representatives were cautiously optimistic and said there are tentative indications of CRE recovery.
Others, such as the head of Europe at LaSalle Investment Management, Philip La Pierre, could have been more positive. He reportedly said:
There’s a lot of hot air being pushed through the Croisette. So you’ve got to navigate that quite carefully.
Rising borrowing costs and post-pandemic open offices cast a shadow on property investments. Reuters reported that year-on-year European commercial capital values dropped by 13.9% in the last quarter of 2023. La Pierre opined that about 30% of European office space is a waste.
Don’t miss out the latest news, subscribe to LeapRate’s newsletter
The US commercial property sector mirrored the European situation. An 11 March 2024 MSCI report indicated that deteriorating office prices placed a yoke on the performance of the entire property market. This report did, however, note the uptick in the European hotel market.
The post MIPIM 2024 Reflects Mixed Feelings on CRE Recovery appeared first on LeapRate.
real estate pandemic recovery european europeInternational
Net Zero, The Digital Panopticon, & The Future Of Food
Net Zero, The Digital Panopticon, & The Future Of Food
Authored by Colin Todhunter via Off-Guardian.org,
The food transition, the energy…

Authored by Colin Todhunter via Off-Guardian.org,
The food transition, the energy transition, net-zero ideology, programmable central bank digital currencies, the censorship of free speech and clampdowns on protest. What’s it all about? To understand these processes, we need to first locate what is essentially a social and economic reset within the context of a collapsing financial system.
Writer Ted Reece notes that the general rate of profit has trended downwards from an estimated 43% in the 1870s to 17% in the 2000s. By late 2019, many companies could not generate enough profit. Falling turnover, squeezed margins, limited cashflows and highly leveraged balance sheets were prevalent.
Professor Fabio Vighi of Cardiff University has described how closing down the global economy in early 2020 under the guise of fighting a supposedly new and novel pathogen allowed the US Federal Reserve to flood collapsing financial markets (COVID relief) with freshly printed money without causing hyperinflation. Lockdowns curtailed economic activity, thereby removing demand for the newly printed money (credit) in the physical economy and preventing ‘contagion’.
According to investigative journalist Michael Byrant, €1.5 trillion was needed to deal with the crisis in Europe alone. The financial collapse staring European central bankers in the face came to a head in 2019. The appearance of a ‘novel virus’ provided a convenient cover story.
The European Central Bank agreed to a €1.31 trillion bailout of banks followed by the EU agreeing to a €750 billion recovery fund for European states and corporations. This package of long-term, ultra-cheap credit to hundreds of banks was sold to the public as a necessary programme to cushion the impact of the pandemic on businesses and workers.
In response to a collapsing neoliberalism, we are now seeing the rollout of an authoritarian great reset — an agenda that intends to reshape the economy and change how we live.
SHIFT TO AUTHORITARIANISM
The new economy is to be dominated by a handful of tech giants, global conglomerates and e-commerce platforms, and new markets will also be created through the financialisation of nature, which is to be colonised, commodified and traded under the notion of protecting the environment.
In recent years, we have witnessed an overaccumulation of capital, and the creation of such markets will provide fresh investment opportunities (including dodgy carbon offsetting Ponzi schemes) for the super-rich to park their wealth and prosper.
This great reset envisages a transformation of Western societies, resulting in permanent restrictions on fundamental liberties and mass surveillance. Being rolled out under the benign term of a ‘Fourth Industrial Revolution’, the World Economic Forum (WEF) says the public will eventually ‘rent’ everything they require (remember the WEF video ‘you will own nothing and be happy’?): stripping the right of ownership under the guise of a ‘green economy’ and underpinned by the rhetoric of ‘sustainable consumption’ and ‘climate emergency’.
Climate alarmism and the mantra of sustainability are about promoting money-making schemes. But they also serve another purpose: social control.
Neoliberalism has run its course, resulting in the impoverishment of large sections of the population. But to dampen dissent and lower expectations, the levels of personal freedom we have been used to will not be tolerated. This means that the wider population will be subjected to the discipline of an emerging surveillance state.
To push back against any dissent, ordinary people are being told that they must sacrifice personal liberty in order to protect public health, societal security (those terrible Russians, Islamic extremists or that Sunak-designated bogeyman George Galloway) or the climate. Unlike in the old normal of neoliberalism, an ideological shift is occurring whereby personal freedoms are increasingly depicted as being dangerous because they run counter to the collective good.
The real reason for this ideological shift is to ensure that the masses get used to lower living standards and accept them. Consider, for instance, the Bank of England’s chief economist Huw Pill saying that people should ‘accept’ being poorer. And then there is Rob Kapito of the world’s biggest asset management firm BlackRock, who says that a “very entitled” generation must deal with scarcity for the first time in their lives.
At the same time, to muddy the waters, the message is that lower living standards are the result of the conflict in Ukraine and supply shocks that both the war and ‘the virus’ have caused.
The net-zero carbon emissions agenda will help legitimise lower living standards (reducing your carbon footprint) while reinforcing the notion that our rights must be sacrificed for the greater good. You will own nothing, not because the rich and their neoliberal agenda made you poor but because you will be instructed to stop being irresponsible and must act to protect the planet.
NET-ZERO AGENDA
But what of this shift towards net-zero greenhouse gas emissions and the plan to slash our carbon footprints? Is it even feasible or necessary?
Gordon Hughes, a former World Bank economist and current professor of economics at the University of Edinburgh, says in a new report that current UK and European net-zero policies will likely lead to further economic ruin.
Apparently, the only viable way to raise the cash for sufficient new capital expenditure (on wind and solar infrastructure) would be a two decades-long reduction in private consumption of up to 10 per cent. Such a shock has never occurred in the last century outside war; even then, never for more than a decade.
But this agenda will also cause serious environmental degradation. So says Andrew Nikiforuk in the article The Rising Chorus of Renewable Energy Skeptics, which outlines how the green techno-dream is vastly destructive.
He lists the devastating environmental impacts of an even more mineral-intensive system based on renewables and warns:
“The whole process of replacing a declining system with a more complex mining-based enterprise is now supposed to take place with a fragile banking system, dysfunctional democracies, broken supply chains, critical mineral shortages and hostile geopolitics.”
All of this assumes that global warming is real and anthropogenic. Not everyone agrees. In the article Global warming and the confrontation between the West and the rest of the world, journalist Thierry Meyssan argues that net zero is based on political ideology rather than science. But to state such things has become heresy in the Western countries and shouted down with accusations of ‘climate science denial’.
Regardless of such concerns, the march towards net zero continues, and key to this is the United Nations Agenda 2030 for Sustainable Development Goals.
Today, almost every business or corporate report, website or brochure includes a multitude of references to ‘carbon footprints’, ‘sustainability’, ‘net zero’ or ‘climate neutrality’ and how a company or organisation intends to achieve its sustainability targets. Green profiling, green bonds and green investments go hand in hand with displaying ‘green’ credentials and ambitions wherever and whenever possible.
It seems anyone and everyone in business is planting their corporate flag on the summit of sustainability. Take Sainsbury’s, for instance. It is one of the ‘big six’ food retail supermarkets in the UK and has a vision for the future of food that it published in 2019.
Here’s a quote from it:
“Personalised Optimisation is a trend that could see people chipped and connected like never before. A significant step on from wearable tech used today, the advent of personal microchips and neural laces has the potential to see all of our genetic, health and situational data recorded, stored and analysed by algorithms which could work out exactly what we need to support us at a particular time in our life. Retailers, such as Sainsbury’s could play a critical role to support this, arranging delivery of the needed food within thirty minutes — perhaps by drone.”
Tracked, traced and chipped — for your own benefit. Corporations accessing all of our personal data, right down to our DNA. The report is littered with references to sustainability and the climate or environment, and it is difficult not to get the impression that it is written so as to leave the reader awestruck by the technological possibilities.
However, the promotion of a brave new world of technological innovation that has nothing to say about power — who determines policies that have led to massive inequalities, poverty, malnutrition, food insecurity and hunger and who is responsible for the degradation of the environment in the first place — is nothing new.
The essence of power is conveniently glossed over, not least because those behind the prevailing food regime are also shaping the techno-utopian fairytale where everyone lives happily ever after eating bugs and synthetic food while living in a digital panopticon.
FAKE GREEN
The type of ‘green’ agenda being pushed is a multi-trillion market opportunity for lining the pockets of rich investors and subsidy-sucking green infrastructure firms and also part of a strategy required to secure compliance required for the ‘new normal’.
It is, furthermore, a type of green that plans to cover much of the countryside with wind farms and solar panels with most farmers no longer farming. A recipe for food insecurity.
Those investing in the ‘green’ agenda care first and foremost about profit. The supremely influential BlackRock invests in the current food system that is responsible for polluted waterways, degraded soils, the displacement of smallholder farmers, a spiralling public health crisis, malnutrition and much more.
It also invests in healthcare — an industry that thrives on the illnesses and conditions created by eating the substandard food that the current system produces. Did Larry Fink, the top man at BlackRock, suddenly develop a conscience and become an environmentalist who cares about the planet and ordinary people? Of course not.
Any serious deliberations on the future of food would surely consider issues like food sovereignty, the role of agroecology and the strengthening of family farms — the backbone of current global food production.
The aforementioned article by Andrew Nikiforuk concludes that, if we are really serious about our impacts on the environment, we must scale back our needs and simplify society.
In terms of food, the solution rests on a low-input approach that strengthens rural communities and local markets and prioritises smallholder farms and small independent enterprises and retailers, localised democratic food systems and a concept of food sovereignty based on self-sufficiency, agroecological principles and regenerative agriculture.
It would involve facilitating the right to culturally appropriate food that is nutritionally dense due to diverse cropping patterns and free from toxic chemicals while ensuring local ownership and stewardship of common resources like land, water, soil and seeds.
That’s where genuine environmentalism and the future of food begins.
Government
Five Aerospace Investments to Buy as Wars Worsen Copy
Five aerospace investments to buy as wars worsen give investors a chance to acquire shares of companies focused on fortifying national defense. The five…
Five aerospace investments to buy as wars worsen give investors a chance to acquire shares of companies focused on fortifying national defense.
The five aerospace investments to buy provide military products to help protect freedom amid Russia’s ongoing onslaught against Ukraine that began in February 2022, as well as supply arms in the Middle East used after Hamas militants attacked and murdered civilians in Israel on Oct. 7. Even though the S&P 500 recently reached all-time highs, these five aerospace investments have remained reasonably priced and rated as recommendations by seasoned analysts and a pension fund chairman.
State television broadcasts in Russia show the country’s soldiers advancing further into Ukrainian territory, but protests have occurred involving family members of those serving in perilous conditions in the invasion of their neighboring nation to be brought home. Even though hundreds of thousands of Russians also have fled to other countries to avoid compulsory military service, the aggressor’s President Vladimir Putin has vowed to continue to send additional soldiers into the fierce fighting.
While Russia’s land-grab of Crimea and other parts of Ukraine show no end in sight, Israel’s war with Hamas likely will last for at least additional months, according to the latest reports. United Nations’ leaders expressed alarm on Dec. 26 about intensifying Israeli attacks that killed more than 100 Palestinians over two days in part of the Gaza Strip, when 15 members of the Israel Defense Force (IDF) also lost their lives.
Five Aerospace Investments to Buy as Wars Worsen: General Dynamics
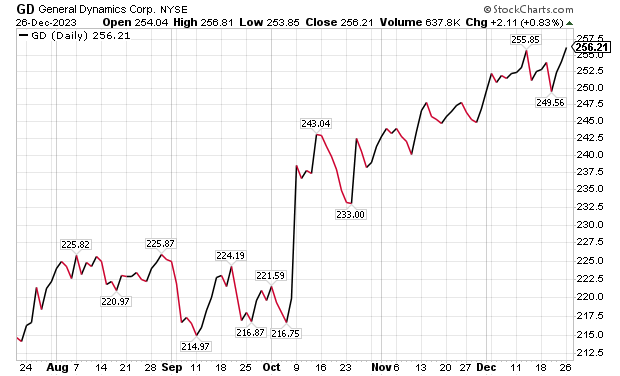
One of the five aerospace investments to buy as wars worsen is General Dynamics (NYSE: GD), a Reston, Virginia-based aerospace company with more than 100,000 employees in 70-plus countries. A key business unit of General Dynamics is Gulfstream Aerospace Corporation, a manufacturer of business aircraft. Other segments of General Dynamics focus on making military products such as Abrams tanks, Stryker fighting vehicles, ASCOD fighting vehicles like the Spanish PIZARRO and British AJAX, LAV-25 Light Armored Vehicles and Flyer-60 lightweight tactical vehicles.
For the U.S. Navy and other allied armed forces, General Dynamics builds Virginia-class attack submarines, Columbia-class ballistic missile submarines, Arleigh Burke-class guided missile destroyers, Expeditionary Sea Base ships, fleet logistics ships, commercial cargo ships, aircraft and naval gun systems, Hydra-70 rockets, military radios and command and control systems. In addition, the company provides radio and optical telescopes, secure mobile phones, PIRANHA and PANDUR wheeled armored vehicles and mobile bridge systems.
Chicago-based investment firm William Blair & Co. is among those recommending General Dynamics. The Chicago firm gave an “outperform” rating to General Dynamics in a Dec. 21 research note.
Gulfstream is seeking G700 FAA certification by the end of 2023, suggesting potentially positive news in the next 10 days, William Blair wrote in its recent research note. The investment firm projected that General Dynamics would trade upward upward upon the G700’s certification.
“General Dynamics’ 2023 aircraft delivery guidance of approximately 134 planes assumes that 19 G700s are delivered in the fourth quarter,” wrote William Blair’s aerospace and defense analyst Louie DiPalma. “Even if deliveries fall short of this target, we believe investors will take a glass-half-full approach upon receipt of the certification.”
Chart courtesy of www.stockcharts.com.
Five Aerospace Investments to Buy as Wars Worsen: GD Outlook
The G700 is a major focus area for investors because it is Gulfstream’s most significant aircraft introduction since the iconic G650 in 2012, DiPalma wrote. Gulfstream has the highest market share in the long-range jet segment of the private aircraft market, the highest profit margin of aircraft peers and the most premium business aviation brand, he added.
“The aircraft remains immensely popular today with corporations and high-net-worth individuals,” Di Palma wrote. “Elon Musk has reportedly placed an order for a G700 to go along with his existing G650. Qatar Airways announced at the Paris Air Show that 10 G700 aircraft will become part of its fleet.”
G700 deliveries and subsequent G800 deliveries are expected to be the cornerstone of Gulfstream’s growth and margin expansion for the next decade, DiPalma wrote. This should lead to a rebound in the stock price as the margins for the G700 and G800 are very attractive, he added.
Management’s guidance is for the aerospace operating margin to increase from about 13.2% in 2022 to roughly 14.0% in 2023 and 15.8% in 2024. Longer term, a high-teens profit margin appears within reach, DiPalma projected.
In other General Dynamics business segments, William Blair expects several yet-unannounced large contract awards for General Dynamics IT, to go along with C$1.7 billion, or US$1.29 billion, in General Dynamics Mission Systems contracts announced on Dec. 20 for the Canadian Army. General Dynamics shares are poised to have a strong 2024, William Blair wrote.
Five Aerospace Investments to Buy as Wars Worsen: VSE Corporation
Alexandria, Virginia-based VSE Corporation’s (NASDAQ: VSEC) price-to-earnings (P/E) valuation multiple of 22 received support when AAR Corp. (NYSE: AIR), a Wood Dale, Illinois, provider of aviation services, announced on Dec. 21 that it would acquire the product support business of Triumph Group (NYSE: TGI), a Berwyn, Pennsylvania, supplier of aerospace services, structures and systems. AAR’s purchase price of $725 million reflects confidence in a continued post-pandemic aerospace rebound.
VSE, a provider of aftermarket distribution and repair services for land, sea and air transportation assets used by government and commercial markets, is rated “outperform” by William Blair. The company’s core services include maintenance, repair and operations (MRO), parts distribution, supply chain management and logistics, engineering support, as well as consulting and training for global commercial, federal, military and defense customers.
“Robust consumer travel demand and aging aircraft fleets have driven elevated maintenance visits,” William Blair’s DiPalma wrote in a Dec. 21 research note. “The AAR–Triumph deal is valued at a premium 13-times 2024 EBITDA multiple, which was in line with the valuation multiple that Heico (NYSE: HEI) paid for Wencor over the summer.”
VSE currently trades at a discounted 9.5 times consensus 2024 earnings before interest, taxes, depreciation and amortization (EBITDA) estimates, as well as 11.6 times consensus 2023 EBITDA.
Five Aerospace Investments to Buy as Wars Worsen: VSE Undervalued?
“We expect that VSE shares will trend higher as investors process this deal,” DiPalma wrote. “VSE shares trade at 9.5 times consensus 2024 adjusted EBITDA, compared with peers and M&A comps in the 10-to-14-times range. We think that VSE’s multiple will expand as it closes the divestiture of its federal and defense business and makes strategic acquisitions. We see consistent 15% annual upside for shares as VSE continues to take share in the $110 billion aviation aftermarket industry.”
William Blair reaffirmed its “outperform” rating for VSE on Dec. 21. The main risk to VSE shares is lumpiness associated with its aviation services margins, Di Palma wrote. However, he raised 2024 estimates to further reflect commentary from VSE’s analysts’ day in November.

Chart courtesy of www.stockcharts.com.
Five Aerospace Investments to Buy as Wars Worsen: HEICO Corporation
HEICO Corporation (NYSEL: HEI), is a Hollywood, Florida-based technology-driven aerospace, industrial, defense and electronics company that also is ranked as an “outperform” investment by William Blair’s DiPalma. The aerospace aftermarket parts provider recently reported fourth-quarter financials above consensus analysts’ estimates, driven by 20% organic growth in HEICO’s flight support group.
HEICO’s management indicated that the performance of recently acquired Wencor is exceeding expectations. However, HEICO leaders offered color on 2024 organic growth and margin expectations that forecast reduced gains. Even though consensus estimates already assumed slowing growth, it is still not a positive for HEICO, DiPalma wrote.
William Blair forecasts 15% annual upside to HEICO’s shares, based on EBITDA growth. HEICO’s management cited a host of reasons for its quarterly outperformance, highlighted by the continued commercial air travel recovery. The company also referenced new product introductions and efficiency initiatives.
HEICO’s defense product sales increased by 26% sequentially, marking the third consecutive sequential increase in defense product revenue. The company’s leaders conveyed that defense in general is moving in the right direction to enhance financial performance.

Chart courtesy of www.stockcharts.com.
Five Dividend-paying Defense and Aerospace Investments to Purchase: XAR
A fourth way to obtain exposure to defense and aerospace investments is through SPDR S&P Aerospace and Defense ETF (XAR). That exchange-traded fund tracks the S&P Aerospace & Defense Select Industry Index. The fund is overweight in industrials and underweight in technology and consumer cyclicals, said Bob Carlson, a pension fund chairman who heads the Retirement Watch investment newsletter.

Bob Carlson, who heads Retirement Watch, answers questions from Paul Dykewicz.
XAR has 34 securities, and 44.2% of the fund is in the 10 largest positions. The fund is up 25.82% in the last 12 months, 22.03% in the past three months and 7.92% for the last month. Its dividend yield recently measured 0.38%.
The largest positions in the fund recently were Axon Enterprise (NASDAQ: AXON), Boeing (NYSE: BA), L3Harris Technologies (NYSE: LHX), Spirit Aerosystems (NYSE: SPR) and Virgin Galactic (NYSE: SPCE).

Chart courtesy of www.stockcharts.com
Five Dividend-paying Defense and Aerospace Investments to Purchase: PPA
The second fund recommended by Carlson is Invesco Aerospace & Defense ETF (PPA), which tracks the SPADE Defense Index. It has the same underweighting and overweighting as XAR, he said.
PPA recently held 52 securities and 53.2% of the fund was in its 10 largest positions. With so many holdings, the fund offers much reduced risk compared to buying individual stocks. The largest positions in the fund recently were Boeing (NYSE: BA), RTX Corp. (NYSE: RTX), Lockheed Martin (NYSE: LMT), Northrop Grumman (NYSE: NOC) and General Electric (NYSE:GE).
The fund is up 19.07% for the past year, 50.34% in the last three months and 5.30% during the past month. The dividend yield recently touched 0.69%.

Chart courtesy of www.stockcharts.com
Other Fans of Aerospace
Two fans of aerospace stocks are Mark Skousen, PhD, and seasoned stock picker Jim Woods. The pair team up to head the Fast Money Alert advisory service They already are profitable in their recent recommendation of Lockheed Martin (NYSE: LMT) in Fast Money Alert.

Mark Skousen, a scion of Ben Franklin, meets with Paul Dykewicz.

Jim Woods, a former U.S. Army paratrooper, co-heads Fast Money Alert.
Bryan Perry, who heads the Cash Machine investment newsletter and the Micro-Cap Stock Trader advisory service, recommends satellite services provider Globalstar (NYSE American: GSAT), of Covington, Louisiana, that has jumped 50.00% since he advised buying it two months ago. Perry is averaging a dividend yield of 11.14% in his Cash Machine newsletter but is breaking out with the red-hot recommendation of Globalstar in his Micro-Cap Stock Trader advisory service.

Bryan Perry heads Cash Machine, averaging an 11.14% dividend yield.
Military Equipment Demand Soars amid Multiple Wars
The U.S. military faces an acute need to adopt innovation, to expedite implementation of technological gains, to tap into the talents of people in various industries and to step-up collaboration with private industry and international partners to enhance effectiveness, U.S. Joint Chiefs of Staff Gen. Charles Q. Brown Jr. told attendees on Nov 16 at a national security conference. Prime examples of the need are showed by multiple raging wars, including the Middle East and Ukraine. A cold war involves China and its increasingly strained relationships with Taiwan and other Asian nations.
The shocking Oct. 7 attack by Hamas on Israel touched off an ongoing war in the Middle East, coupled with Russia’s February 2022 invasion and continuing assault of neighboring Ukraine. Those brutal military conflicts show the fragility of peace when determined aggressors are willing to use any means necessary to achieve their goals. To fend off such attacks, rapid and effective response is required.
“The Department of Defense is doing more than ever before to deter, defend, and, if necessary, defeat aggression,” Gen. Brown said at the National Security Innovation Forum at the Johns Hopkins University Bloomberg Center in Washington, D.C.
One of Russia’s war ships, the 360-foot-long Novocherkassk, was damaged on Dec. 26 by a Ukrainian attack on the Black Sea port of Feodosia in Crimea. This video of an explosion at the port that reportedly shows a section of the ship hit by aircraft-guided missiles.

Chairman Joint Chiefs of Staff Gen. Charles Q. Brown, Jr.
Photo By: Benjamin Applebaum
National security threats can compel immediate action, Gen. Brown said he quickly learned since taking his post on Oct. 1.
“We may not have much warning when the next fight begins,” Gen. Brown said. “We need to be ready.”
In a pre-recorded speech at the national security conference, Michael R. Bloomberg, founder of Bloomberg LP, told the John Hopkins national security conference attendees about the critical need for collaboration between government and industry.
“Building enduring technological advances for the U.S. military will help our service members and allies defend freedom across the globe,” Bloomberg said.
The “horrific terrorist attacks” against Israel and civilians living there on Oct. 7 underscore the importance of that mission, Bloomberg added.
Paul Dykewicz, www.pauldykewicz.com, is an accomplished, award-winning journalist who has written for Dow Jones, the Wall Street Journal, Investor’s Business Daily, USA Today, the Journal of Commerce, Seeking Alpha, Guru Focus and other publications and websites. Attention Holiday Gift Buyers! Consider purchasing Paul’s inspirational book, “Holy Smokes! Golden Guidance from Notre Dame’s Championship Chaplain,” with a foreword by former national championship-winning football coach Lou Holtz. The uplifting book is great gift and is endorsed by Joe Montana, Joe Theismann, Ara Parseghian, “Rocket” Ismail, Reggie Brooks, Dick Vitale and many others. Call 202-677-4457 for special pricing on multiple-book purchases or autographed copies! Follow Paul on Twitter @PaulDykewicz. He is the editor of StockInvestor.com and DividendInvestor.com, a writer for both websites and a columnist. He further is editorial director of Eagle Financial Publications in Washington, D.C., where he edits monthly investment newsletters, time-sensitive trading alerts, free e-letters and other investment reports. Paul previously served as business editor of Baltimore’s Daily Record newspaper, after writing for the Baltimore Business Journal and Crain Communications.
The post Five Aerospace Investments to Buy as Wars Worsen Copy appeared first on Stock Investor.
dow jones sp 500 nasdaq stocks pandemic etf micro-cap army recovery russia ukraine china-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International6 days ago
International6 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International6 days ago
International6 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges






















