International
Is Aphria A Better Cannabis Bet Than Canopy Growth?
Is Aphria A Better Cannabis Bet Than Canopy Growth?
Cannabis sales in Canada have been on a rise since May following a decline in April. As per Statistics Canada, sales of legal adult-use cannabis in Canada grew by 5.2% month-over-month to C$244.9 million in August. Cannabis is legal in Canada for both medical and recreational purposes. Moreover, with Cannabis 2.0, cannabis derivative products like edibles, vapes, concentrates, and beverages have also become legal in the country.
However, intense competition from the illegal market continues to impact the sales of Canadian cannabis companies. Meanwhile, companies are awaiting the legalization of marijuana at the Federal level in the US to capture potential growth opportunities. Against this backdrop, we will use the TipRanks Stock Comparison tool to see if Aphria or Canopy Growth offers a more compelling investment opportunity.

Aphria (APHA)
Ontario-based Aphria sells its medical cannabis products under its namesake brand Aphria and Broken Coast and adult-use or recreational cannabis products under the Solei, RIFF, Good Supply as well as the Broken Coast brands.
Last year, Aphria’s co-founder Vic Neufeld resigned as the CEO of the company amid allegations by short-sellers that the company had overpaid for the acquisition of LATAM Holdings and that insiders had profited from the deal. Vic Neufeld was succeeded by Irwin Simon and under his leadership, Aphria has moved ahead of the controversy and improved its operational performance. It has generated positive adjusted EBITDA for six consecutive quarters unlike its rivals Canopy Growth and Aurora Cannabis, which are still not profitable on an adjusted EBITDA basis.
However, the company’s recently reported 1Q FY21 (ended Aug. 31) results disappointed investors and caused an 18% drop in its shares on Oct. 15. Aphria’s 1Q revenue grew 16% Y/Y to C$145.7 million but was down 4% from the prior quarter due to lower distribution revenue from its German distributor CC Pharma because of the pandemic.
The company’s adjusted EBITDA came in at C$10 million in 1Q FY21, up from C$8.6 million in 4Q FY20 and C$1 million in 1Q FY20. However, investors were concerned as the company slipped into a GAAP net loss per share of C$0.02 in 1Q FY21 compared to EPS of C$0.07 in 1Q FY20. (See APHA stock analysis on TipRanks)
Looking forward, Aphria intends to launch new cannabis 2.0 derivative products, further strengthen its position in the vapes market where it is already the market leader and focus on the newly launched B!NGO brand (a large format, economy brand utilizing lower potency cannabis). It also aims to grow further in international markets by catering to the demand for either GACP (Good Agricultural and Collection Practices) or EU GMP (European Union Good Manufacturing Practices) certified products.
Commenting about the decline in stock following the 1Q results, Haywood analyst Neal Gilmer said, “Investors seemed focused on a drop in the distribution revenue and that the bulk of cannabis growth was due to sell-in of its new value brand. We remain positive on Aphria’s strong market share across its brand portfolio.”
“We continue to view Aphria as the leader in the Canadian LP [Licensed Producer] landscape. We believe yesterday’s 18 per cent correction provides an attractive entry point given Aphria’s strong market share position. We expect the company will continue to demonstrate its leading position, supported by expanding EBITDA margins in fiscal 2021,” added Gilmer in a research note to investors.
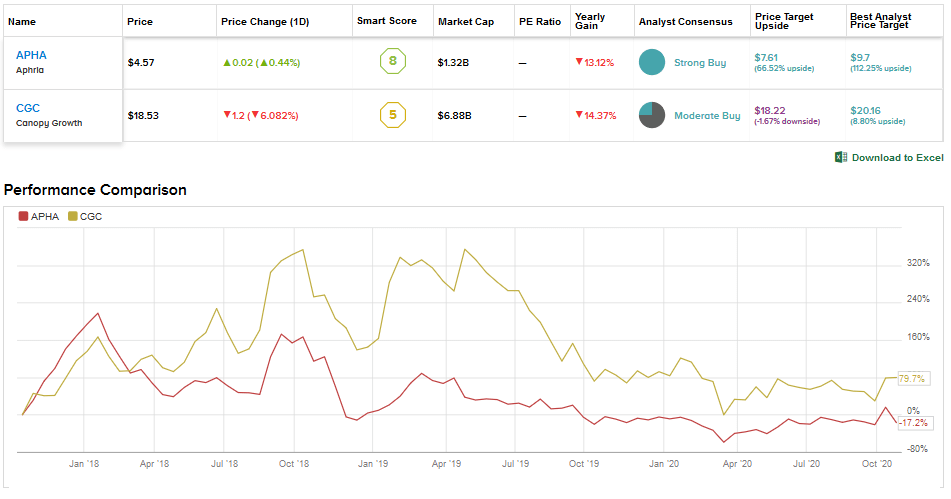
Indeed, the Street is very bullish about Aphria and has a Strong Buy consensus based on 7 unanimous Buy ratings. With shares down 12.3% year-to-date, the average analyst price target of $7.61 indicates robust upside potential of 66.5% over the coming months.

Canopy Growth (CGC)
Canopy Growth is one of the largest producers of cannabis and sells its medical and recreational cannabis products through brands like Tweed, Spectrum Therapeutics, Houseplant, DNA Genetics, Tokyo Smoke, Doja, Van der Pop and Maitri. The company’s investors have been concerned about its aggressive investments in production facilities, acquisitions and growth initiatives being a drag on its bottom line. Canopy Growth has not yet posted a positive adjusted EBITDA.
To improve its profitability, the company has been implementing several restructuring initiatives with a focus on reducing costs and cash burn rate. It has also been curbing its capacity at certain plants while shutting down others. Canopy Growth was able to bring down its adjusted EBITDA loss to C$92.2 million in 1Q FY21 (ended Jun. 30) compared to a loss of C$93.4 million in the prior-year quarter.
Meanwhile, 1Q FY21 revenue grew 22% Y/Y to C$110.4 million on strength in the company's medical cannabis business. However, revenue grew by just 2% Q/Q due to lower Canadian recreational cannabis revenue reflecting the impact of COVID-19 and heightened competition in dried flower-based products.
Canopy sees huge growth opportunities in the derivatives markets, including vapes, chocolates and beverages, and continues to expand its portfolio through innovative products. It has a strong presence in the Canadian cannabis-infused beverage space with a market share of 74% through products like Tweed’s Houndstooth & Soda and Bakerstreet & Ginger. Canopy is now gearing up to launch its beverages in the US market in summer next year through a partnership with Acreage holdings.
Its other strategic initiatives for the US market include the launch of a new e-commerce site shopcanopy.com, expansion of the First & Free brand into topical and creams and further penetration of the BioSteel (Canopy is a major stakeholder in BioSteel) sports beverages.
Last month, Canopy launched Martha Stewart CBD – a new line of hemp-derived wellness supplements (like gummies, softgels and oil drops), which are specially formulated by celebrity Martha Stewart. (See CGC stock analysis on TipRanks)
Recently, Cantor Fitzgerald analyst Pablo Zuanic reiterated his Hold rating for Canopy and increased his price target to $20.93 from $20.56. The analyst noted that like its rival Aurora Cannabis, Canopy is also significantly dependent on the flower value segment rather than higher-margin products. As a result, both the companies are adversely impacted by price deflation, even at the low end of the price scale.
However, Zuanic feels that Canopy has much more financial flexibility than Aurora and can afford to take more risks. He sees "near-term upside" for Canopy shares based on encouraging market data and expects 24% sequential sales growth for Canopy in 2Q FY21, which is over three times the 7% consensus analyst estimate.
Meanwhile, the Street is cautiously optimistic about Canopy Growth with a Moderate Buy consensus based on 2 Buys versus 6 Holds and no Sells. The $18.22 average analyst price target indicates a possible downside of 1.7% ahead. Shares have already declined 12.2% year-to-date.

Conclusion
Aphria has been consistently delivering positive adjusted EBITDA over the recent quarters while Canopy Growth is still away from that goal. Currently, Aphria appears to be a better cannabis pick than Canopy Growth as reflecting in the Street’s highly bullish stance and upside potential ahead.
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment
The post Is Aphria A Better Cannabis Bet Than Canopy Growth? appeared first on TipRanks Financial Blog.
International
EyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
Ramiro Ribeiro
After six years as head of clinical development at Apellis Pharmaceuticals, Ramiro Ribeiro is joining EyePoint Pharmaceuticals as CMO.
“The…

After six years as head of clinical development at Apellis Pharmaceuticals, Ramiro Ribeiro is joining EyePoint Pharmaceuticals as CMO.
“The retinal community is relatively small, so everybody knows each other,” Ribeiro told Endpoints News in an interview. “As soon as I started to talk about EyePoint, I got really good feedback from KOLs and physicians on its scientific standards and quality of work.”
Ribeiro kicked off his career as a clinician in Brazil, earning a doctorate in stem cell therapy for retinal diseases. He previously held roles at Alcon and Ophthotech Corporation, now known as Astellas’ M&A prize Iveric Bio.
At Apellis, Ribeiro oversaw the Phase III development, filing and approval of Syfovre, the first drug for geographic atrophy secondary to age-related macular degeneration (AMD). The complement C3 inhibitor went on to make $275 million in 2023 despite reports of a rare side effect that only emerged after commercialization.
Now, Ribeiro is hoping to replicate that success with EyePoint’s lead candidate, EYP-1901 for wet AMD, which is set to enter the Phase III LUGANO trial in the second half of the year after passing a Phase II test in December.
Ribeiro told Endpoints he was optimistic about the company’s intraocular sustained-delivery tech, which he said could help address treatment burden and compliance issues seen with injectables. He also has plans to expand the EyePoint team.
“My goal is not just execution of the Phase III study — of course that’s a priority — but also looking at the pipeline and which different assets we can bring in to leverage the strength of the team that we have,” Ribeiro said.
— Ayisha Sharma
 Remco Steenbergen
Remco Steenbergen→ Sandoz CFO Colin Bond will retire on June 30 and board member Remco Steenbergen will replace him. Steenbergen, who will step down from the board when he takes over on July 1, had a 20-year career with Philips and has held the group CFO post at Deutsche Lufthansa since January 2021. Bond joined Sandoz nearly two years ago and is the former finance chief at Evotec and Vifor Pharma. Investors didn’t react warmly to Wednesday’s news as shares fell by almost 4%.
The Swiss generics and biosimilars company, which finally split from Novartis in October 2023, has also nominated FogPharma CEO Mathai Mammen to the board of directors. The ex-R&D chief at J&J will be joined by two other new faces, Swisscom chairman Michael Rechsteiner and former Unilever CFO Graeme Pitkethly.
On Monday, Sandoz said it completed its $70 million purchase of Coherus BioSciences’ Lucentis biosimilar Cimerli sooner than expected. The FDA then approved its first two biosimilars of Amgen’s denosumab the next day, in a move that could whittle away at the pharma giant’s market share for Prolia and Xgeva.
 Sean Marett
Sean Marett→ BioNTech’s chief business and commercial officer Sean Marett will retire on July 1 and will have an advisory role “until the end of the year,” the German drugmaker said in a release. Legal chief James Ryan will assume CBO responsibilities and BioNTech plans to name a new chief commercial officer by the end of the month. Marett was hired as BioNTech’s COO in 2012 after gigs at GSK, Evotec and Next Pharma, and led its commercial efforts as the Pfizer-partnered Comirnaty received the first FDA approval for a Covid-19 vaccine. BioNTech has also built a cancer portfolio that TD Cowen’s Yaron Werber described as “one of the most extensive” in biotech, from antibody-drug conjugates to CAR-T therapies.
 Chris Austin
Chris Austin→ GSK has plucked Chris Austin from Flagship and he’ll start his new gig as the pharma giant’s SVP, research technologies on April 1. After a long career at NIH in which he was director of the National Center for Advancing Translational Sciences (NCATS), Austin became CEO of Flagship’s Vesalius Therapeutics, which debuted with a $75 million Series A two years ago this week but made job cuts that affected 43% of its employees six months into the life of the company. In response to Austin’s departure, John Mendlein — who chairs the board at Sail Biomedicines and has board seats at a few other Flagship biotechs — will become chairman and interim CEO at Vesalius “later this month.”
→ BioMarin has lined up Cristin Hubbard to replace Jeff Ajer as chief commercial officer on May 20. Hubbard worked for new BioMarin chief Alexander Hardy as Genentech’s SVP, global product strategy, immunology, infectious diseases and ophthalmology, and they had been colleagues for years before Hardy was named Genentech CEO in 2019. She shifted to Roche Diagnostics as global head of partnering in 2021 and had been head of global product strategy for Roche’s pharmaceutical division since last May. Sales of the hemophilia A gene therapy Roctavian have fallen well short of expectations, but Hardy insisted in a recent investor call that BioMarin is “still very much at the early stage” in the launch.
 Pilar de la Rocha
Pilar de la Rocha→ BeiGene has promoted Pilar de la Rocha to head of Europe, global clinical operations. After 13 years in a variety of roles at Novartis, de la Rocha was named global head of global clinical operations excellence at the Brukinsa maker in the summer of 2022. A short time ago, BeiGene ended its natural killer cell therapy alliance with Shoreline Biosciences, saying that it was “a result of BeiGene’s internal prioritization decisions and does not reflect any deficit in Shoreline’s platform technology.”
 Andy Crockett
Andy Crockett→ Andy Crockett has resigned as CEO of KalVista Pharmaceuticals. Crockett had been running the company since its launch in 2011 and will hand the keys to president Ben Palleiko, who joined KalVista in 2016 as CFO. Serious safety issues ended a Phase II study of its hereditary angioedema drug KVD824, but KalVista is mounting a comeback with positive Phase III results for sebetralstat in the same indication and could compete with Takeda’s injectable Firazyr. “If approved, sebetralstat may offer a compelling treatment option for patients and their caregivers given the long-standing preference for an effective and safe oral therapy that provides rapid symptom relief for HAE attacks,” Crockett said last month.
 Steven Lo
Steven Lo→ Vaxart has tapped Steven Lo as its permanent president and CEO, while interim chief Michael Finney will stay on as chairman. Endpoints News last caught up with Lo when he became CEO at Valitor, the UC Berkeley spinout that raised a $28 million Series B round in October 2022. The ex-Zosano Pharma CEO had a handful of roles in his 13 years at Genentech before his appointments as chief commercial officer of Corcept Therapeutics and Puma Biotechnology. Andrei Floroiu resigned as Vaxart’s CEO in mid-January.
 Kartik Krishnan
Kartik Krishnan→ Kartik Krishnan has taken over for Martin Driscoll as CEO of OncoNano Medicine, and Melissa Paoloni has moved up to COO at the cancer biotech located in the Dallas-Fort Worth suburb of Southlake. The execs were colleagues at Arcus Biosciences, Gilead’s TIGIT partner: Krishnan spent two and a half years in the CMO post, while Paoloni was VP of corporate development and external alliances. In 2022, Krishnan took the CMO job at OncoNano and was just promoted to president and head of R&D last November. Paoloni came on board as OncoNano’s SVP, corporate development and strategy not long after Krishnan’s first promotion.
→ Genesis Research Group, a consultancy specializing in market access, has brought in David Miller as chairman and CEO, replacing co-founder Frank Corvino — who is transitioning to the role of vice chairman and senior advisor. Miller joins the New Jersey-based team with a number of roles under his belt from Biogen (SVP of global market access), Elan (VP of pharmacoeconomics) and GSK (VP of global health outcomes).
 Adrian Schreyer
Adrian Schreyer→ Adrian Schreyer helped build Exscientia’s AI drug discovery platform from the ground up, but he has packed his bags for Nimbus Therapeutics’ AI partner Anagenex. The new chief technology officer joined Exscientia in 2013 as head of molecular informatics and was elevated to technology chief five years later. He then held the role of VP, AI technology until January, a month before Exscientia fired CEO Andrew Hopkins.
→ Paul O’Neill has been promoted from SVP to EVP, quality & operations, specialty brands at Mallinckrodt. Before his arrival at the Irish pharma in March 2023, O’Neill was executive director of biologics operations in the second half of his 12-year career with Merck driving supply strategy for Keytruda. Mallinckrodt’s specialty brands portfolio includes its controversial Acthar Gel (a treatment for flares in a number of chronic and autoimmune indications) and the hepatorenal syndrome med Terlivaz.
 David Ford
David Ford→ Staying in Ireland, Prothena has enlisted David Ford as its first chief people officer. Ford worked in human resources at Sanofi from 2002-17 and then led the HR team at Intercept, which was sold to Italian pharma Alfasigma in late September. We recently told you that Daniel Welch, the former InterMune CEO who was a board member at Intercept for six years, will succeed Lars Ekman as Prothena’s chairman.
 Ben Stephens
Ben Stephens→ Co-founded by Sanofi R&D chief Houman Ashrafian and backed by GSK, Eli Lilly partner Sitryx stapled an additional $39 million to its Series A last fall. It has now welcomed a pair of execs: Ben Stephens (COO) had been finance director for ViaNautis Bio and Rinri Therapeutics, and Gordon Dingwall (head of clinical operations) is a Roche and AstraZeneca vet who led development operations at Mission Therapeutics. Dingwall has also served as a clinical operations leader for Shionogi and Freeline Therapeutics.
 Steve Alley
Steve Alley→ MBrace Therapeutics, an antibody-drug conjugate specialist that nabbed $85 million in Series B financing last November, has named Steve Alley as CSO. Alley spent two decades at Seagen before the $43 billion buyout by Pfizer and was the ADC maker’s executive director, translational sciences.
→ California cancer drug developer Apollomics, which has been mired in Nasdaq compliance problems nearly a year after it joined the public markets through a SPAC merger, has recruited Matthew Plunkett as CFO. Plunkett has held the same title at Nkarta as well as Imago BioSciences — leading the companies to $290 million and $155 million IPOs, respectively — and at Aeovian Pharmaceuticals since March 2022.
 Heinrich Haas
Heinrich Haas→ Co-founded by Oxford professor Adrian Hill — the co-inventor of AstraZeneca’s Covid-19 vaccine — lipid nanoparticle biotech NeoVac has brought in Heinrich Haas as chief technology officer. During his nine years at BioNTech, Haas was VP of RNA formulation and drug delivery.
 Kimberly Lee
Kimberly Lee→ New Jersey-based neuro biotech 4M Therapeutics is making its Peer Review debut by introducing Kimberly Lee as CBO. Lee was hired at Taysha Gene Therapies during its meteoric rise in 2020 and got promoted to chief corporate affairs officer in 2022. Earlier, she led corporate strategy and investor relations efforts for Lexicon Pharmaceuticals.
→ Another Peer Review newcomer, Osmol Therapeutics, has tapped former Exelixis clinical development chief Ron Weitzman as interim CMO. Weitzman only lasted seven months as medical chief of Tango Therapeutics after Marc Rudoltz had a similarly short stay in that position. Osmol is going after chemotherapy-induced peripheral neuropathy and chemotherapy-induced cognitive impairment with its lead asset OSM-0205.
→ Last August, cardiometabolic disease player NeuroBo Pharmaceuticals locked in Hyung Heon Kim as president and CEO. Now, the company is giving Marshall Woodworth the title of CFO and principal financial and accounting officer, after he served in the interim since last October. Before NeuroBo, Woodworth had a string of CFO roles at Nevakar, Braeburn Pharmaceuticals, Aerocrine and Fureix Pharmaceuticals.
 Claire Poll
Claire Poll→ Claire Poll has retired after more than 17 years as Verona Pharma’s general counsel, and the company has appointed Andrew Fisher as her successor. In his own 17-year tenure at United Therapeutics that ended in 2018, Fisher was chief strategy officer and deputy general counsel. The FDA will decide on Verona’s non-cystic fibrosis bronchiectasis candidate ensifentrine by June 26.
 Nancy Lurker
Nancy Lurker→ Alkermes won its proxy battle with Sarissa Capital Management and is tinkering with its board nearly nine months later. The newest director, Bristol Myers Squibb alum Nancy Lurker, ran EyePoint Pharmaceuticals from 2016-23 and still has a board seat there. For a brief period, Lurker was chief marketing officer for Novartis’ US subsidiary.
→ Chaired by former Celgene business development chief George Golumbeski, Shattuck Labs has expanded its board to nine members by bringing in ex-Seagen CEO Clay Siegall and Tempus CSO Kate Sasser. Siegall holds the top spots at Immunome and chairs the board at Tourmaline Bio, while Sasser came to Tempus from Genmab in 2022.
 Scott Myers
Scott Myers→ Ex-AMAG Pharmaceuticals and Rainier Therapeutics chief Scott Myers has been named chairman of the board at Convergent Therapeutics, a radiopharma player that secured a $90 million Series A last May. Former Magenta exec Steve Mahoney replaced Myers as CEO of Viridian Therapeutics a few months ago.
→ Montreal-based Find Therapeutics has elected Tony Johnson to the board of directors. Johnson is in his first year as CEO of Domain Therapeutics. He is also the former chief executive at Goldfinch Bio, the kidney disease biotech that closed its doors last year.
 Habib Dable
Habib Dable→ Former Acceleron chief Habib Dable has replaced Kala Bio CEO Mark Iwicki as chairman of the board at Aerovate Therapeutics, which is signing up patients for Phase IIb and Phase III studies of its lead drug AV-101 for pulmonary arterial hypertension. Dable joined Aerovate’s board in July and works part-time as a venture partner for RA Capital Management.
 Julie Cherrington
Julie Cherrington→ In the burgeoning world of ADCs, Elevation Oncology is developing one of its own that targets Claudin 18.2. Its board is now up to eight members with the additions of Julie Cherrington and Mirati CMO Alan Sandler. Cherrington, a venture partner at Brandon Capital Partners, also chairs the boards at Actym Therapeutics and Tolremo Therapeutics. Sandler took the CMO job at Mirati in November 2022 and will stay in that position after Bristol Myers acquired the Krazati maker.
 Patty Allen
Patty Allen→ Lonnie Moulder’s Zenas BioPharma has welcomed Patty Allen to the board of directors. Allen was a key figure in Vividion’s $2 billion sale to Bayer as the San Diego biotech’s CFO, and she’s a board member at Deciphera Pharmaceuticals, SwanBio Therapeutics and Anokion.
→ In January 2023, Y-mAbs Therapeutics cut 35% of its staff to focus on commercialization of Danyelza. This week, the company has reserved a seat on its board of directors for Nektar Therapeutics CMO Mary Tagliaferri. Tagliaferri also sits on the boards of Enzo Biochem and is a former board member of RayzeBio.
→ The ex-Biogen neurodegeneration leader at the center of Aduhelm’s controversial approval is now on the scientific advisory board at Asceneuron, a Swiss-based company focused on Alzheimer’s and Parkinson’s. Samantha Budd-Haeberlein tops the list of new SAB members, which also includes Henrik Zetterberg, Rik Ossenkoppele and Christopher van Dyck.
nasdaq covid-19 vaccine treatment fda therapy rna brazil europeInternational
Deflationary pressures in China – be careful what you wish for
Until recently, China’s decelerating inflation was welcomed by the West, as it led to lower imported prices and helped reduce inflationary pressures….

Until recently, China’s decelerating inflation was welcomed by the West, as it led to lower imported prices and helped reduce inflationary pressures. However, China’s consumer prices fell for the third consecutive month in December 2023, delaying the expected rebound in economic activity following the lifting of COVID-19 controls. For calendar year 2023, CPI growth was negligible, whilst the producer price index declined by 3.0 per cent.
China’s inflation dynamics

Chinese consumers are hindered by the weaker residential property market and high youth unemployment. Several property developers have defaulted, collectively wiping out nearly all the U.S.$155 billion worth of U.S. dollar denominated-bonds.
Meanwhile, the Shanghai Composite Index is at half of its record high, recorded in late 2007. The share prices of major developers, including Evergrande Group, Country Garden Holdings, Sunac China and Shimao Group, have declined by an average of 98 per cent over recent years. Some economists are pointing to the Japanese experience of a debt-deflation cycle in the 1990s, with economic stagnation and elevated debt levels.
Australia has certainly enjoyed the “pull-up effect” from China, particularly with the iron-ore price jumping from around U.S.$20/tonne in 2000 to an average closer to U.S.$120/tonne over the 17 years from 2007. With strong volume increases, the value of Australia’s iron ore exports has jumped 20-fold to around A$12 billion per month, accounting for approximately 35 per cent of Australia’s exports.
For context, China takes 85 per cent of Australia’s iron ore exports, whilst Australia accounts for 65 per cent of China’s iron ore imports. China’s steel industry depends on its own domestic iron ore mines for 20 per cent of its requirement, however, these are high-cost operations and need high iron ore prices to keep them in business. To reduce its dependence on Australia’s iron ore, China has increased its use of scrap metal and invested large sums of money in Africa, including the Simandou mine in Guinea, which is forecast to export 60 million tonnes of iron ore from 2028.
The Chinese housing market has historically been the source of 40 per cent of China’s steel usage. However, the recent high iron ore prices are attributable to the growth in China’s industrial and infrastructure activity, which has offset the weakness in residential construction.
Whilst this has continued to deliver supernormal profits for Australia’s major iron ore producers (and has greatly assisted the federal budget), watch out for any sustainable downturn in the iron ore price, particularly if the deflationary pressures in China continue into the medium term.
unemployment covid-19 bonds shanghai composite housing market africa chinaInternational
Deterra Royalties half-yearly result: stable performance and growth Initiatives
Deterra Royalties (ASX:DRR) was established through a strategic demerger from Iluka Resources Ltd (ASX:ILU) in 2020. At the core of Deterra Royalties portfolio…

Deterra Royalties (ASX:DRR) was established through a strategic demerger from Iluka Resources Ltd (ASX:ILU) in 2020. At the core of Deterra Royalties portfolio lies long-life, Mining Area C (MAC), a premier iron ore mining operation in the Pilbara region of Western Australia, operationally managed by BHP. This key asset is underpinned by a royalty agreement that ensures Deterra Royalties receives quarterly payments equivalent to 1.232 per cent of the revenue generated, alongside substantial one-off payments of A$1 million for each dry metric tonne increase in annual production capacity.
South flank, a critical component of the MAC, exemplifies BHP’s latest advancement in iron ore mining, marking its inaugural production in May 2021. In financial year 2023, MAC annual iron ore production amounted to 126 million wet metric tonnes, up 14 per cent on the prior year. The company has reiterated that capacity payments have been set at 118 million tonnes last year and are expected to be updated to current production of 126 million tonnes in June 2024, with potential upside to 145 million tonnes shortly after that. Thus, there is potential upside to dividends of $8 million in capacity payments by June 2024. Meanwhile, revenue amounted to $215.2 million plus a $13 million capacity payment from south flank expansion. Net profit after tax came in at $152.5 million.
The company distributes 100 per cent of its profits as dividends.
In a global landscape marked by burgeoning uncertainty and China’s post-COVID-19 economic malaise, Deterra Royalties emerges as providing iron exposure with greater stability. Deterra Royalties offers investors exposure to the iron ore market with distinctly reduced volatility compared to traditional mining entities.
With that background established, the company released its half-yearly results for FY24, reporting figures that were largely in line with both internal expectations and market consensus. The company continues to explore avenues for portfolio expansion, particularly in bulk, base, and battery commodity royalties, although no deals have been finalised. With substantial undrawn debt facilities of $500 million and recent declines in junior mining company stocks, Deterra Royalties may be moving closer to securing new deals to create new royalties or purchase existing royalties.
Deterra Royalties reported a net profit after tax (NPAT) of $78.7 million for the first half of FY24, matching internal projections and closely aligning with market estimates, albeit slightly below consensus by three per cent. The declared dividend of $14.89 conditions precedent, representing 100 per cent of NPAT in accordance with Deterra Royalties dividend policy, also fell within anticipated ranges but slightly missed consensus. Revenue for the period stood at A$119 million, consistent with the pre-reported royalty revenue update.
Operating costs dipped by two per cent from the previous half-year to A$4.3 million but were up by four per cent year-on-year. Notably, business development costs surged to A$1.3 million, marking a 50 per cent increase from the previous period and a 140 per cent rise from the same period last year. This uptick reflects Deterra Royalties intensified efforts to evaluate growth opportunities, as managing director Julian Andrews highlighted.
Deterra Royalties remains steadfast in its pursuit of growth opportunities, maintaining a flexible approach in both the size and type of investments/royalties sought. The company’s focus spans non-precious metals, including bulk, base, and battery metals, primarily targeting developed mining jurisdictions across Australia, North America, South America, and Europe. Deterra Royalties continues to prioritise royalties for production or near-production companies.
A company that pays 100 per cent of its earnings as a dividend is relatively easy to value with a discounted cash flow (DCF). Adopting a required return of 6-7 per cent of the weighted average cost of capital (WACC), Deterra Royalties valuation falls in a range between A$4.70 and $5.10 per share.
In summary, Deterra Royalties’ half-yearly results provided the stable and somewhat predictable operational performance our portfolio managers value, whilst also providing iron ore exposure.
The Montgomery Fund and the Montgomery [Private] Fund owns shares in Dettera Royalties. This blog was prepared 19 February 2024 with the information we have today, and our view may change. It does not constitute formal advice or professional investment advice. If you wish to trade Deterra Royalties, you should seek financial advice.
stocks covid-19 south america europe china-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 month ago
International1 month agoWar Delirium
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges



















