Government
68% Of Americans Believe Joe Biden Acted Illegally Or Unethically In Hunter’s Foreign Dealings; New Poll Finds
68% Of Americans Believe Joe Biden Acted Illegally Or Unethically In Hunter’s Foreign Dealings; New Poll Finds
Authored by Jonathan Turley,
A…

A new AP-NORC poll is out and, consistent with earlier polling results, shows that there remains a disconnect between the media coverage and public opinion over the Biden corruption scandal. Despite the continued dismissal or downplaying of the scandal by many in the media, a massive 68 percent of Americans believe that President Biden acted either illegally or unethically in his involvement in his son’s foreign dealings.
The largest group (36%) believe that the president has done something illegal. The second largest group (33%) believed Biden has done something unethical.
Only 30% of American adults believe Biden has “not done anything wrong” as he has repeatedly claimed. It seems like the same 30% has remained supportive on every issue for Biden — the same unchanging support that we have found at the extremes of every poll for both Biden and Trump.
Notably, however, 40% of Democrats now believe Biden has done something illegal or unethical in his handling of his son’s business dealings.
The percentage is overwhelming among Republicans at 96%.
What should worry Biden is that 74% of the key independent vote believes that he has done something illegal or unethical.
What is most striking about these polls is that the public has reached these conclusions despite a media that is overwhelmingly protective of the President. Most major media outlets downplayed or ignored the Hunter Biden laptop story while adopting ever-changing narratives to excuse the President’s role.
Just this week, the Boston Globe ran a story titled “Attacking Biden, GOP tries to have it both ways; He’s old and feeble — and a master criminal?” Of course, you do not have to be a genius to engage in influence peddling. You just need to be corrupt. Menendez was no Brainiac in taking cash and cars from foreign sources. Moreover, one of Hunter Biden’s corrupt clients reportedly viewed him as dumber than his dog but still gave him millions. The labyrinth of shell companies and accounts were set up by others, not the President or his son.
Despite the media running cover for the Bidens for years, the public is just not buying it. Indeed, they are not buying the media. The Washington Post recently acknowledged that it will lose $100 million and announced a new round of layoffs or buyouts.
CEO Patty Stonesifer blamed the continuing loss of readers and revenues on being “overly optimistic” about its growth in readership, subscriptions and ads for the past two years. There is a continued effort at failing media outlets to blame their losses on everything other than their embrace of advocacy journalism and writing off half of the country with its strident political bias.
In fairness to Stonsifer, advertising revenues are down for newspapers with the rise of digital sources for the news. However, the embrace of non-traditional sources of news is not, in my view, entirely due to technological or platform changes.
Much like some companies pursuing woke agendas despite opposition from their consumers, media executives cannot acknowledge that their brand of journalism may be at fault. Editors at the Post and other leading outlets have rejected objectivity in favor of advocacy for journalists — blurring the line between reporting and commentary. That was evident recently when the Post publicly reaffirmed that it was standing by the false reporting of Philip Bump on a variety of disproven stories. These are conspiracy theories and false claims that have been long debunked from Lafayette Park to the Hunter Biden laptop but the Post just declared that Bump was correct.
For his part, Bump recently became irate when confronted with his past false claims, declaring “I just I’m gonna lose my mind. I’m gonna lose my mind.”
Notably, the interviewer explained that many do not believe his reporting and Bump dismissed them as uninformed and said that they need to listen to him as the expert on such matters.
Noam Dworman asked “is there nothing we can talk about … half the country believes this stuff.”
Bump: “I know, because half the country doesn’t actually dig into the issues.”
Dworman: “Here’s your chance to disabuse people. They don’t read the Washington Post.”
Bump responded: “There’s just no point, because all you want to do is you want to have me here as the putative expert so that you can present me with things that have been debunked multiple times that I’ve written about.”
Dworman: “What’s been debunked?”
Bump: “These, these claims. I’ve written about this, this argument about his dad calling him. I’ve written about this. Did you read what I wrote?”
Dworman: “It’s not debunked. Neither of us were there.”
Bump: “Well, I debunked it in the standpoint that I’ve already addressed this and presented the counterarguments to it.”
Before leaving, Bump explained that there was little value to explaining his past claims “because you don’t listen to the press. I’m sitting here and I’m telling you, you’re wrong about these things, and you don’t listen, and you continue to insist upon things that are, you know, parsing of language. And it’s just, it’s this is why I keep saying it’s silly.”
Once again, the Post recently stood by Bump’s past false claims.
This is why the Stonesifer’s account is so striking. She assured staff that “we are working to find ways to return our business to a healthier place in the coming year.” That “healthier place” could be with more balanced reporters.
There are still excellent journalists at the Post who can restore balance in its coverage and regain the trust (and business) of readers. However, it requires a bit of self-awareness and reexamination on the part of the owner and the editors. Instead, the Post continues to write for that same 30% revealed in the poll by the AP, which steadfastly supports the Bidens despite rising evidence of corrupt influence peddling and false statements. You cannot sustain a major publication on 30% of the readership while writing off any conservatives or independents who want balanced coverage.
The disconnect in these polls shows that many in the public are simply dismissing the common narrative of the media. The uniformity in much of the coverage, particularly in the Biden corruption scandal, leaves many with the feeling of a de facto state media. That appearance is not helped with the White House giving marching orders to the media on how to attack the investigations into the Biden family while powerful Democrats warn reporters to “back off” the Bidens.
I have been a columnist and a television analyst for roughly three decades. I have written for papers like the Washington Post and worked for NBC, CBS, BBC, and Fox. I care deeply for the future of these media outlets. However, the lack of impact of the media on public opinion reflects the record low levels of trust in the media found in numerous polls.
That is the result, in my opinion, of the embrace of advocacy journalism and echo chamber coverage.
The “healthier place” for the media is the very place that many reporters abandoned in the past with the tradition of objectivity and neutrality in journalism.
Government
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
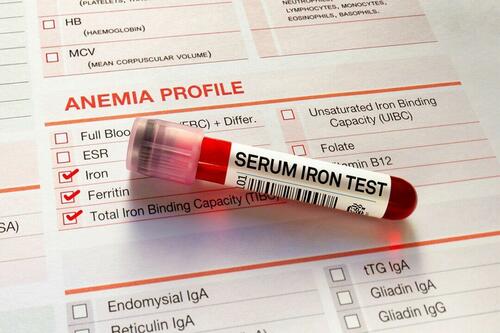
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Government
Walmart joins Costco in sharing key pricing news
The massive retailers have both shared information that some retailers keep very close to the vest.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trumpGovernment
Walmart has really good news for shoppers (and Joe Biden)
The giant retailer joins Costco in making a statement that has political overtones, even if that’s not the intent.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trump-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















