These 2 Penny Stocks Are Poised for a Massive Rally, Says Roth Capital
These 2 Penny Stocks Are Poised for a Massive Rally, Says Roth Capital


Bull or bear market, no investment is a sure thing. Especially in the current financial environment, which remains riddled with uncertainty, finding compelling plays can be challenging for even the most seasoned market watchers. However, this is not to say that investment opportunities with stand-out growth prospects can’t be found.
Roth Capital research analyst Zegbeh Jallah pointed to the healthcare sector, in particular, as an area of the market worthy of investor attention.
“Biotech had had a strong performance during the midst of the pandemic, and we expect it to remain so, largely driven by solid fundamental catalysts. This is supported by the resumption of preclinical and clinical efforts at many companies, albeit with some new procedures in place,” Jallah noted.
Bearing this in mind, our focus turned to two penny stocks from the healthcare space backed by Roth Capital. Along with the stamp of approval, the firm believes that both of these tickers trading for less than $5 per share are primed for a massive rally.
Digging a bit deeper, we used TipRanks’ database to find out what makes both so compelling despite the risk involved.
Seelos Therapeutics (SEEL)
Primarily focused on neurological and psychiatric disorders, Seelos Therapeutics works to bring cutting-edge therapies to market. Currently going for $0.67 apiece, Roth Capital believes that its share price presents investors with an opportunity to get in on the action.
Calling SEEL “substantially undervalued,” firm analyst Jonathan Aschoff points to two of the company's pivotal programs, SLS-002 (intranasal racemic ketamine), its treatment for acute suicidal ideation and behavior (ASIB) in patients with major depressive disorder (MDD), and SLS-005 (trehalose), its ALS treatment, as major upside drivers.
SLS-002 is set to enter a proof-of-concept (POC) trial, with the FDA “eager for a useful anti-suicide drug.” Aschoff noted, “We firmly believe that, although it is formally called a POC trial, achievement of two endpoints, the primary endpoint of Montgomery-Åsberg depression rating scale (MADRS) and the key secondary endpoint of Sheehan-suicidality tracking scale clinically meaningful change measure (S-STS CMCM), will be enough to allow SEEL to file for approval of a ketamine importantly differentiated by its ability to reduce suicidal ideation.”
Aschoff believes it’s likely that SEEL will have already kicked off the 120-patient randomized trial portion when it publishes data from 16 patients dosed with SLS-002. The analyst added, “We also believe that SEEL was given extremely helpful trial design guidance from the FDA due to the clearly evident observation that current national and global affairs are contributing to suicide more strongly now than perhaps ever.”
As for SLS-005, the company recently got permission from the FDA to proceed with its registrational Phase 2b/3 ALS trial. Based on data from multiple preclinical studies, treatment with the therapy resulted in the preservation of motor neurons, motor function and prolonged survival.
What’s more, SLS-005 has been granted Orphan Drug Designation (ODD) in the U.S. and E.U. for other indications such as Sanfilippo syndrome, spinocerebellar ataxia type 3 (SCA3) and oculopharyngeal muscular dystrophy (OPMD), as well as Fast Track designation for OPMD.
"We believe that a trial in SCA3 is more likely to come first [...] We anticipate SEEL's SCA3 trial to enroll about 150 patients and evaluate patients by SARA at six months, in which showing stability would be meaningful and showing improvement would be a home run," Aschoff wrote.
To this end, Aschoff rates SEEL a Buy along with a $12 price target. This puts the upside potential at a massive 1,715%. (To watch Aschoff’s track record, click here)
Turning now to the rest of the Street, 2 Buys and no Holds or Sells have been published in the last three months. Therefore, SEEL has a Moderate Buy consensus rating. With the average price target clocking in at $8, the upside potential lands at 1,111%. (See SEEL stock analysis on TipRanks)

Acer Therapeutics (ACER)
Developing therapies for rare and life-threatening diseases, Acer Therapeutics wants to address the unmet medical needs of patients. With several catalysts slated for the near-term, Roth Capital thinks its $2.50 share price reflects an attractive entry point.
Analyst Jonathan Aschoff, who also covers SEEL for the firm, points out that during a recent meeting with management, the “overwhelmingly focus was on emetine, ACER-001 and Edsivo, and each of these programs are capable of providing at least one meaningful near-term investment catalyst.”
Emetine, a potential COVID-19 treatment, is being developed as part of a collaboration with the National Center for Advancing Translational Sciences (NCATS), one of the National Institutes of Health (NIH). Speaking to the therapy’s potential, Aschoff notes that the candidate reactivates the cellular stress response, making it a strong antiviral with nanomolar potency about 50 times greater than remdesivir, Gilead’s COVID-19 treatment. The broad antiviral activity could also allow the drug to be used against future novel viruses, in the analyst’s opinion.
ACER is pursuing BARDA and Gates Foundation funding for the program. While Aschoff acknowledges that the company could fund part of the clinical development itself, he argues the program is more likely to progress with external funding. The capital would allow ACER to submit an IND in 1H21 and initiate a Phase 2/3 trial later that year. As a response from BARDA is set to come in Q3 2020, the analyst sees a major possible catalyst.
As for ACER-001, its fully taste-masked, immediate-release formulation of sodium phenylbutyrate (NaPB) developed using a microencapsulation process, Aschoff believes ACER is speaking to several larger pharmaceutical companies about partnership opportunities, with plenty of upside in store should an agreement be reached.
“We highly favor ACER-001's edge over the competition, given Buphenyl's awful taste and smell and Ravicti's egregious pricing of about $900,000 per year. With ACER-001's taste masking and parity pricing to Buphenyl (about $350,000 per year), ACER-001 is a no brainer, in our view...We believe that FDA buy in on the food effect without requiring addition clinical work would be a meaningful stock catalyst,” the analyst commented.
Additionally, Edsivo, ACER’s new chemical entity (NCE) designed for the treatment of vEDS, reflects a key point of strength, in Aschoff’s opinion. Ehlers-Danlos Syndrome (EDS) is a group of hereditary disorders of connective tissue. “We essentially view Edsivo as a call option, with significant share price upside if the FDA allows ACER to amend and refile its NDA after only having to include existing natural history data... Edsivo could generate meaningful U.S. revenue for ACER, about $350 million annually, if approved for vascular Ehlers-Danlos syndrome,” he explained.
It should come as no surprise, then, that Aschoff stayed with the bulls. To this end, he left a Buy rating and $10 price target on the stock. Investors could be pocketing a gain of 300%, should this target be met in the twelve months ahead.
Looking at the consensus breakdown, opinions are more varied. As 2 Buys and 3 Holds have been issued in the last three months, the word on the Street is that ACER is a Moderate Buy. At $10, the average price target matches Aschoff’s. (See ACER stock analysis on TipRanks)
To find good ideas for penny stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.
The post These 2 Penny Stocks Are Poised for a Massive Rally, Says Roth Capital appeared first on TipRanks Financial Blog.
Government
Problems After COVID-19 Vaccination More Prevalent Among Naturally Immune: Study
Problems After COVID-19 Vaccination More Prevalent Among Naturally Immune: Study
Authored by Zachary Stieber via The Epoch Times (emphasis…
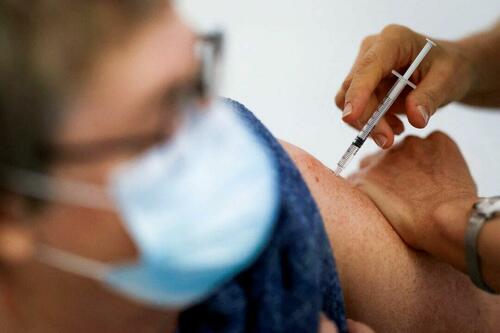
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
People who recovered from COVID-19 and received a COVID-19 shot were more likely to suffer adverse reactions, researchers in Europe are reporting.
Participants in the study were more likely to experience an adverse reaction after vaccination regardless of the type of shot, with one exception, the researchers found.
Across all vaccine brands, people with prior COVID-19 were 2.6 times as likely after dose one to suffer an adverse reaction, according to the new study. Such people are commonly known as having a type of protection known as natural immunity after recovery.
People with previous COVID-19 were also 1.25 times as likely after dose 2 to experience an adverse reaction.
The findings held true across all vaccine types following dose one.
Of the female participants who received the Pfizer-BioNTech vaccine, for instance, 82 percent who had COVID-19 previously experienced an adverse reaction after their first dose, compared to 59 percent of females who did not have prior COVID-19.
The only exception to the trend was among males who received a second AstraZeneca dose. The percentage of males who suffered an adverse reaction was higher, 33 percent to 24 percent, among those without a COVID-19 history.
“Participants who had a prior SARS-CoV-2 infection (confirmed with a positive test) experienced at least one adverse reaction more often after the 1st dose compared to participants who did not have prior COVID-19. This pattern was observed in both men and women and across vaccine brands,” Florence van Hunsel, an epidemiologist with the Netherlands Pharmacovigilance Centre Lareb, and her co-authors wrote.
There were only slightly higher odds of the naturally immune suffering an adverse reaction following receipt of a Pfizer or Moderna booster, the researchers also found.
The researchers performed what’s known as a cohort event monitoring study, following 29,387 participants as they received at least one dose of a COVID-19 vaccine. The participants live in a European country such as Belgium, France, or Slovakia.
Overall, three-quarters of the participants reported at least one adverse reaction, although some were minor such as injection site pain.
Adverse reactions described as serious were reported by 0.24 percent of people who received a first or second dose and 0.26 percent for people who received a booster. Different examples of serious reactions were not listed in the study.
Participants were only specifically asked to record a range of minor adverse reactions (ADRs). They could provide details of other reactions in free text form.
“The unsolicited events were manually assessed and coded, and the seriousness was classified based on international criteria,” researchers said.
The free text answers were not provided by researchers in the paper.
“The authors note, ‘In this manuscript, the focus was not on serious ADRs and adverse events of special interest.’” Yet, in their highlights section they state, “The percentage of serious ADRs in the study is low for 1st and 2nd vaccination and booster.”
Dr. Joel Wallskog, co-chair of the group React19, which advocates for people who were injured by vaccines, told The Epoch Times: “It is intellectually dishonest to set out to study minor adverse events after COVID-19 vaccination then make conclusions about the frequency of serious adverse events. They also fail to provide the free text data.” He added that the paper showed “yet another study that is in my opinion, deficient by design.”
Ms. Hunsel did not respond to a request for comment.
She and other researchers listed limitations in the paper, including how they did not provide data broken down by country.
The paper was published by the journal Vaccine on March 6.
The study was funded by the European Medicines Agency and the Dutch government.
No authors declared conflicts of interest.
Some previous papers have also found that people with prior COVID-19 infection had more adverse events following COVID-19 vaccination, including a 2021 paper from French researchers. A U.S. study identified prior COVID-19 as a predictor of the severity of side effects.
Some other studies have determined COVID-19 vaccines confer little or no benefit to people with a history of infection, including those who had received a primary series.
The U.S. Centers for Disease Control and Prevention still recommends people who recovered from COVID-19 receive a COVID-19 vaccine, although a number of other health authorities have stopped recommending the shot for people who have prior COVID-19.
Another New Study
In another new paper, South Korean researchers outlined how they found people were more likely to report certain adverse reactions after COVID-19 vaccination than after receipt of another vaccine.
The reporting of myocarditis, a form of heart inflammation, or pericarditis, a related condition, was nearly 20 times as high among children as the reporting odds following receipt of all other vaccines, the researchers found.
The reporting odds were also much higher for multisystem inflammatory syndrome or Kawasaki disease among adolescent COVID-19 recipients.
Researchers analyzed reports made to VigiBase, which is run by the World Health Organization.
“Based on our results, close monitoring for these rare but serious inflammatory reactions after COVID-19 vaccination among adolescents until definitive causal relationship can be established,” the researchers wrote.
The study was published by the Journal of Korean Medical Science in its March edition.
Limitations include VigiBase receiving reports of problems, with some reports going unconfirmed.
Funding came from the South Korean government. One author reported receiving grants from pharmaceutical companies, including Pfizer.
Uncategorized
Key shipping company files for Chapter 11 bankruptcy
The Illinois-based general freight trucking company filed for Chapter 11 bankruptcy to reorganize.

The U.S. trucking industry has had a difficult beginning of the year for 2024 with several logistics companies filing for bankruptcy to seek either a Chapter 7 liquidation or Chapter 11 reorganization.
The Covid-19 pandemic caused a lot of supply chain issues for logistics companies and also created a shortage of truck drivers as many left the business for other occupations. Shipping companies, in the meantime, have had extreme difficulty recruiting new drivers for thousands of unfilled jobs.
Related: Tesla rival’s filing reveals Chapter 11 bankruptcy is possible
Freight forwarder company Boateng Logistics joined a growing list of shipping companies that permanently shuttered their businesses as the firm on Feb. 22 filed for Chapter 7 bankruptcy with plans to liquidate.
The Carlsbad, Calif., logistics company filed its petition in the U.S. Bankruptcy Court for the Southern District of California listing assets up to $50,000 and and $1 million to $10 million in liabilities. Court papers said it owed millions of dollars in liabilities to trucking, logistics and factoring companies. The company filed bankruptcy before any creditors could take legal action.
Lawsuits force companies to liquidate in bankruptcy
Lawsuits, however, can force companies to file bankruptcy, which was the case for J.J. & Sons Logistics of Clint, Texas, which on Jan. 22 filed for Chapter 7 liquidation in the U.S. Bankruptcy Court for the Western District of Texas. The company filed bankruptcy four days before the scheduled start of a trial for a wrongful death lawsuit filed by the family of a former company truck driver who had died from drowning in 2016.
California-based logistics company Wise Choice Trans Corp. shut down operations and filed for Chapter 7 liquidation on Jan. 4 in the U.S. Bankruptcy Court for the Northern District of California, listing $1 million to $10 million in assets and liabilities.
The Hayward, Calif., third-party logistics company, founded in 2009, provided final mile, less-than-truckload and full truckload services, as well as warehouse and fulfillment services in the San Francisco Bay Area.
The Chapter 7 filing also implemented an automatic stay against all legal proceedings, as the company listed its involvement in four legal actions that were ongoing or concluded. Court papers reportedly did not list amounts for damages.
In some cases, debtors don't have to take a drastic action, such as a liquidation, and can instead file a Chapter 11 reorganization.
Shutterstock
Nationwide Cargo seeks to reorganize its business
Nationwide Cargo Inc., a general freight trucking company that also hauls fresh produce and meat, filed for Chapter 11 bankruptcy protection in the U.S. Bankruptcy Court for the Northern District of Illinois with plans to reorganize its business.
The East Dundee, Ill., shipping company listed $1 million to $10 million in assets and $10 million to $50 million in liabilities in its petition and said funds will not be available to pay unsecured creditors. The company operates with 183 trucks and 171 drivers, FreightWaves reported.
Nationwide Cargo's three largest secured creditors in the petition were Equify Financial LLC (owed about $3.5 million,) Commercial Credit Group (owed about $1.8 million) and Continental Bank NA (owed about $676,000.)
The shipping company reported gross revenue of about $34 million in 2022 and about $40 million in 2023. From Jan. 1 until its petition date, the company generated $9.3 million in gross revenue.
Related: Veteran fund manager picks favorite stocks for 2024
bankruptcy pandemic covid-19 stocksUncategorized
Key shipping company files Chapter 11 bankruptcy
The Illinois-based general freight trucking company filed for Chapter 11 bankruptcy to reorganize.

The U.S. trucking industry has had a difficult beginning of the year for 2024 with several logistics companies filing for bankruptcy to seek either a Chapter 7 liquidation or Chapter 11 reorganization.
The Covid-19 pandemic caused a lot of supply chain issues for logistics companies and also created a shortage of truck drivers as many left the business for other occupations. Shipping companies, in the meantime, have had extreme difficulty recruiting new drivers for thousands of unfilled jobs.
Related: Tesla rival’s filing reveals Chapter 11 bankruptcy is possible
Freight forwarder company Boateng Logistics joined a growing list of shipping companies that permanently shuttered their businesses as the firm on Feb. 22 filed for Chapter 7 bankruptcy with plans to liquidate.
The Carlsbad, Calif., logistics company filed its petition in the U.S. Bankruptcy Court for the Southern District of California listing assets up to $50,000 and and $1 million to $10 million in liabilities. Court papers said it owed millions of dollars in liabilities to trucking, logistics and factoring companies. The company filed bankruptcy before any creditors could take legal action.
Lawsuits force companies to liquidate in bankruptcy
Lawsuits, however, can force companies to file bankruptcy, which was the case for J.J. & Sons Logistics of Clint, Texas, which on Jan. 22 filed for Chapter 7 liquidation in the U.S. Bankruptcy Court for the Western District of Texas. The company filed bankruptcy four days before the scheduled start of a trial for a wrongful death lawsuit filed by the family of a former company truck driver who had died from drowning in 2016.
California-based logistics company Wise Choice Trans Corp. shut down operations and filed for Chapter 7 liquidation on Jan. 4 in the U.S. Bankruptcy Court for the Northern District of California, listing $1 million to $10 million in assets and liabilities.
The Hayward, Calif., third-party logistics company, founded in 2009, provided final mile, less-than-truckload and full truckload services, as well as warehouse and fulfillment services in the San Francisco Bay Area.
The Chapter 7 filing also implemented an automatic stay against all legal proceedings, as the company listed its involvement in four legal actions that were ongoing or concluded. Court papers reportedly did not list amounts for damages.
In some cases, debtors don't have to take a drastic action, such as a liquidation, and can instead file a Chapter 11 reorganization.
Shutterstock
Nationwide Cargo seeks to reorganize its business
Nationwide Cargo Inc., a general freight trucking company that also hauls fresh produce and meat, filed for Chapter 11 bankruptcy protection in the U.S. Bankruptcy Court for the Northern District of Illinois with plans to reorganize its business.
The East Dundee, Ill., shipping company listed $1 million to $10 million in assets and $10 million to $50 million in liabilities in its petition and said funds will not be available to pay unsecured creditors. The company operates with 183 trucks and 171 drivers, FreightWaves reported.
Nationwide Cargo's three largest secured creditors in the petition were Equify Financial LLC (owed about $3.5 million,) Commercial Credit Group (owed about $1.8 million) and Continental Bank NA (owed about $676,000.)
The shipping company reported gross revenue of about $34 million in 2022 and about $40 million in 2023. From Jan. 1 until its petition date, the company generated $9.3 million in gross revenue.
Related: Veteran fund manager picks favorite stocks for 2024
bankruptcy pandemic covid-19 stocks-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International7 days ago
International7 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International7 days ago
International7 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Spread & Containment2 days ago
Spread & Containment2 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A




















