Spread & Containment
Six Up-and-Coming COVID-19 Vaccines
Besides Pfizer/BioNTech, Moderna, and J&J, many other biopharmas have jabs in the clinic that have shown significant protection against SARS-CoV-2.
The post Six Up-and-Coming COVID-19 Vaccines appeared first on GEN – Genetic Engineering and Biotechnol
Since the start of the COVID-19 pandemic, the response from public health agencies, regulators, researchers, and industry has been dominated by the development of vaccines. As of October 14, McGill University’s McGill COVID19 Vaccine Tracker listed 153 vaccines in clinical development, of which 23 had been approved or authorized for emergency use in at least one country.
In the United States, the FDA has approved one COVID-19 vaccine (Pfizer/BioNTech’s Comirnaty) and authorized for emergency use two others (Moderna’s Moderna COVID-19 Vaccine and Johnson & Johnson’s Janssen COVID-19 Vaccine). Beyond the United States, most approvals and authorizations have been granted to those vaccines and others developed by AstraZeneca/University of Oxford (122 countries), Sinopharm (65), Sinovac Biotech (40), Russia’s Gamaleya Research Institute (16), and CanSino Biologics (9).
Six COVID-19 vaccines that have shown positive data and/or regulatory progress in recent months are highlighted in this article.
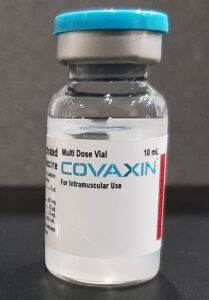
Covaxin
Ocugen (Malvern, PA) is participating in regulatory reviews with the FDA and Health Canada for Covaxin, the vaccine the company is co-developing in both countries with Bharat Biotech (Hyderabad, India). In a preprint posted in July, researchers from Bharat and partners showed Covaxin to have 77.8% efficacy in mild, moderate, and severe COVID-19 disease; 93.4% against severe COVID-19 disease alone; and 65.2% against the Delta variant, in a trial that enrolled 25,798 participants aged 18–98 in India.
“Covaxin is the only product with controlled clinical trial efficacy data on the Delta variant,” Shankar Musunuri, PhD, MBA, Ocugen chairman, CEO, and co-founder, told GEN. The point here is that the data for Covaxin is not limited to neutralization data extrapolated from laboratory studies. (Pfizer/BioNTech, Moderna, and other developers have launched trials of reformulated vaccine candidates designed to generate clinical efficacy data vs. Delta.)
Covaxin is a whole-virion, inactivated COVID-19 vaccine formulated with a toll-like receptor 7/8 agonist molecule (IMDG) and Alhydroxiquim-II, an NIH-funded adjuvant discovered by ViroVax. Covaxin has received emergency authorization in 16 countries including India, with emergency applications pending in more than 60 additional countries.

Chairman of the Board, CEO, and Co-Founder, Ocugen
Ocugen is seeking FDA guidance on pursuing a biologics license application. “As soon as we reach an agreement on how to bridge some additional trials, we’ll do that and work closely with the FDA,” Musunuri said.
Bharat Biotech developed Covaxin with the Indian Council of Medical Research, National Institute of Virology.
GBP510
SK Bioscience (Seongnam-si, South Korea) and GlaxoSmithKline (GSK; Brentford, United Kingdom) launched a Phase III trial of GBP510 in August. For this trial, the plan is to administer GBP510 with GSK’s pandemic adjuvant to more than 4,000 participants.
“Recruitment is progressing well,” a GSK spokesperson told GEN. “Results are expected in the first half of 2022, after which, subject to regulatory approval, we hope to supply the vaccine at scale worldwide through the COVID-19 Vaccines Global Access (COVAX) Facility.”
Earlier this year, SK and GSK reported positive interim Phase I/II results showing a 100% seroconversion rate, with all participants who received the adjuvanted vaccine candidate developing strong neutralizing antibody responses. Neutralizing antibody titers were between five and eight times higher than sera from people who had recovered from COVID-19.
GBP510 combined with GSK’s adjuvant is a self-assembled nanoparticle vaccine candidate targeting the receptor binding domain of the SARS-CoV-2 spike protein. SK is developing the antigen with the Institute for Protein Design at the University of Washington with support from the Bill and Melinda Gates Foundation and the Coalition for Epidemic Preparedness Initiative (CEPI) as part of the Wave 2 vaccine investment project to develop more accessible and affordable COVID-19 vaccines.
INO-4800
Inovio Pharmaceuticals is preparing to launch the global Phase III segment of its Phase II/III INNOVATE trial (NCT04642638) evaluating INO-4800, a DNA vaccine targeting the major surface antigen spike protein of SARS-CoV-2. Interim efficacy results are expected in 2022.

Senior Vice President, R&D
Inovio Pharmaceuticals
Inovio has reported complete maintenance of both CD4+ and CD8+ T-cell responses against all variants of concern tested, including Delta. “The broad T-cell responses that we are generating with 4800 give us confidence that we can protect against the known variants—Alpha, Beta, Gamma, and Delta—but also potentially the as-yet-unknown future variants,” Kate E. Broderick, PhD, senior vice president, R&D, Inovio, told GEN.
INNOVATE will be conducted with Advaccine Biopharmaceuticals Suzhou, which holds exclusive rights to develop, manufacture, and commercialize INO-4800 within Greater China. INNOVATE is set to recruit participants across Latin America, Asia, and Africa (numbers will vary depending on regional infection rate)—though not the United States, where the trial remains on partial clinical hold because the FDA has raised questions about the Cellectra 2000 device used for delivering INO-4800.
“We will be gathering data and look forward to working with the FDA to get off the hold,” Broderick added. Afterward, Inovio plans to file a biologics license application using efficacy from the INNOVATE trial.
NVX-CoV2373
Novavax (Gathersburg, MD) told GEN it expected to seek an emergency use authorization from the FDA for NVX-CoV2373 during the fourth quarter. Outside the United States, Novavax and partner Serum Institute of India have submitted their first emergency use filings in India, Indonesia, and the Philippines, as well as an application for emergency use listing with the World Health Organization. Novavax added that it was working to complete ongoing rolling submissions to the World Health Organization and regulators in Europe, the United Kingdom, Canada, Australia, and New Zealand.
“Our first doses are committed to the developing world, and our largest customer is the COVAX Facility, with a cumulative 1.1 billion doses committed in partnership with the Serum Institute of India,” a Novavax spokesperson said.

Novavax in June reported robust data from its pivotal Phase III PREVENT-19 trial (NCT04611802), which showed 90.4% efficacy against mild, moderate, and severe disease; 100% protection against moderate and severe disease; 91% efficacy among high-risk populations; 92.6% efficacy against variants of concern/interest; and 100% efficacy against variants not classified.
More recently, Novavax launched a clinical study assessing a combination vaccine (NVX-CoV2373 and NanoFlu) against COVID-19 and influenza. “The combination vaccine positions Novavax to address a future need to annually immunize against both SARS-CoV-2 and influenza virus, potentially in advance of the winter transmission season,” Novavax noted. “It will not impact the ongoing and future standalone studies of our COVID-19 and NanoFlu vaccines.”

SCB-2019 (CpG 1018/Alum)
Clover Biopharmaceuticals (Chengdu, China) and Dynavax Technologies (Emeryville, CA) reported in September that their protein-based COVID-19 vaccine candidate SCB-2019 (CpG 1018/Alum), in combination with Dynavax’s CpG 1018 adjuvant, met the primary and secondary efficacy endpoints in the global Phase II/III SPECTRA trial (NCT04672395), a trial that enrolled more than 30,000 participants in the Philippines, Brazil, Colombia, South Africa, and Belgium.
SCB-2019 (CpG 1018/Alum) showed 100% efficacy against severe COVID-19 and hospitalizations, and 84% efficacy against moderate-to-severe COVID-19 caused by any strain of SARS-CoV-2; all strains in the study were of variants. Clover also reported 79% overall efficacy against COVID-19 of any severity caused by Delta, 92% against Gamma, and 59% against Mu.

“Based on our pioneering data, we believe that SCB-2019 (CpG 1018/Alum) could be utilized as an important tool to combat this pandemic,” stated Joshua Liang, CEO, Clover. “We remain dedicated to expediting the availability and equitable access of our COVID-19 vaccine candidate for global distribution.”
The vaccine combines SCB-2019 antigen, a stabilized trimeric form of the S-protein (S-Trimer) based on the original strain of the SARS-CoV-2 virus, with CpG 1018 and alum.
VBI-2902
VBI Vaccines (Cambridge, MA) said in June that its enveloped virus-like particle vaccine VBI-2902 showed proof of concept by generating initial positive data in the Phase Ia portion of a Phase I/II trial (NCT04773665). Data showed that a 5 µg dose expressing an optimized form of the SARS-CoV-2 spike antigen and adjuvanted with aluminum phosphate induced neutralization titers in 100% of participants, with 4.3 times the geometric mean titer (GMT) compared to convalescent sera.

President and CEO, VBI Vaccines
After two doses, VBI-2902 induced antibody binding titers in 100% of participants, with a geometric mean titer of 4,047 units/mL, 5.0 times the geometric mean titer of the convalescent serum panel.
“We achieved a human proof of concept at a very low dose using simply alum, which has been in tens if not hundreds of millions of people,” Jeff Baxter, VBI’s president and CEO, told GEN.
“A two-dose regimen makes sense,” added David E. Anderson, PhD, VBI’s co-founder and chief scientific officer. “In terms of the 5 µg dose, we were very pleased with the kind of potency we saw. We are encouraged by the validating data seen to date from VBI-2902.”

Chief Scientific Officer, VBI Vaccines
VBI-2902 is one of three enveloped virus-like particle vaccine candidates that VBI Vaccines is developing. In September, the company began a clinical study of VBI-2905 as a two-dose course and as a single booster dose against variants of concern including Beta and Delta. “Our biggest concern is understanding the extent to which the VBI-2905 booster can really give us protective immunity across the landscape of variants that are currently circulating,” Anderson noted.
During the first half of 2022, VBI Vaccines expects to launch its first clinical study of its trivalent pancoronavirus vaccine candidate VBI-2901, expressing the SARS-CoV-2, SARS-CoV, and MERS-CoV spike proteins.
The post Six Up-and-Coming COVID-19 Vaccines appeared first on GEN - Genetic Engineering and Biotechnology News.
emergency use authorization governor pandemic covid-19 vaccine fda preclinical genetic dna transmission south korea africa india brazil canada europe russia china world health organizationSpread & Containment
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19…

Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19 vaccination was a mistake due to ethical and other concerns, a top government doctor warned Dr. Anthony Fauci after Dr. Fauci promoted mass vaccination.
“Coercing or forcing people to take a vaccine can have negative consequences from a biological, sociological, psychological, economical, and ethical standpoint and is not worth the cost even if the vaccine is 100% safe,” Dr. Matthew Memoli, director of the Laboratory of Infectious Diseases clinical studies unit at the U.S. National Institute of Allergy and Infectious Diseases (NIAID), told Dr. Fauci in an email.
“A more prudent approach that considers these issues would be to focus our efforts on those at high risk of severe disease and death, such as the elderly and obese, and do not push vaccination on the young and healthy any further.”
Employing that strategy would help prevent loss of public trust and political capital, Dr. Memoli said.
The email was sent on July 30, 2021, after Dr. Fauci, director of the NIAID, claimed that communities would be safer if more people received one of the COVID-19 vaccines and that mass vaccination would lead to the end of the COVID-19 pandemic.
“We’re on a really good track now to really crush this outbreak, and the more people we get vaccinated, the more assuredness that we’re going to have that we’re going to be able to do that,” Dr. Fauci said on CNN the month prior.
Dr. Memoli, who has studied influenza vaccination for years, disagreed, telling Dr. Fauci that research in the field has indicated yearly shots sometimes drive the evolution of influenza.
Vaccinating people who have not been infected with COVID-19, he said, could potentially impact the evolution of the virus that causes COVID-19 in unexpected ways.
“At best what we are doing with mandated mass vaccination does nothing and the variants emerge evading immunity anyway as they would have without the vaccine,” Dr. Memoli wrote. “At worst it drives evolution of the virus in a way that is different from nature and possibly detrimental, prolonging the pandemic or causing more morbidity and mortality than it should.”
The vaccination strategy was flawed because it relied on a single antigen, introducing immunity that only lasted for a certain period of time, Dr. Memoli said. When the immunity weakened, the virus was given an opportunity to evolve.
Some other experts, including virologist Geert Vanden Bossche, have offered similar views. Others in the scientific community, such as U.S. Centers for Disease Control and Prevention scientists, say vaccination prevents virus evolution, though the agency has acknowledged it doesn’t have records supporting its position.
Other Messages
Dr. Memoli sent the email to Dr. Fauci and two other top NIAID officials, Drs. Hugh Auchincloss and Clifford Lane. The message was first reported by the Wall Street Journal, though the publication did not publish the message. The Epoch Times obtained the email and 199 other pages of Dr. Memoli’s emails through a Freedom of Information Act request. There were no indications that Dr. Fauci ever responded to Dr. Memoli.
Later in 2021, the NIAID’s parent agency, the U.S. National Institutes of Health (NIH), and all other federal government agencies began requiring COVID-19 vaccination, under direction from President Joe Biden.
In other messages, Dr. Memoli said the mandates were unethical and that he was hopeful legal cases brought against the mandates would ultimately let people “make their own healthcare decisions.”
“I am certainly doing everything in my power to influence that,” he wrote on Nov. 2, 2021, to an unknown recipient. Dr. Memoli also disclosed that both he and his wife had applied for exemptions from the mandates imposed by the NIH and his wife’s employer. While her request had been granted, his had not as of yet, Dr. Memoli said. It’s not clear if it ever was.
According to Dr. Memoli, officials had not gone over the bioethics of the mandates. He wrote to the NIH’s Department of Bioethics, pointing out that the protection from the vaccines waned over time, that the shots can cause serious health issues such as myocarditis, or heart inflammation, and that vaccinated people were just as likely to spread COVID-19 as unvaccinated people.
He cited multiple studies in his emails, including one that found a resurgence of COVID-19 cases in a California health care system despite a high rate of vaccination and another that showed transmission rates were similar among the vaccinated and unvaccinated.
Dr. Memoli said he was “particularly interested in the bioethics of a mandate when the vaccine doesn’t have the ability to stop spread of the disease, which is the purpose of the mandate.”
The message led to Dr. Memoli speaking during an NIH event in December 2021, several weeks after he went public with his concerns about mandating vaccines.
“Vaccine mandates should be rare and considered only with a strong justification,” Dr. Memoli said in the debate. He suggested that the justification was not there for COVID-19 vaccines, given their fleeting effectiveness.
Julie Ledgerwood, another NIAID official who also spoke at the event, said that the vaccines were highly effective and that the side effects that had been detected were not significant. She did acknowledge that vaccinated people needed boosters after a period of time.
The NIH, and many other government agencies, removed their mandates in 2023 with the end of the COVID-19 public health emergency.
A request for comment from Dr. Fauci was not returned. Dr. Memoli told The Epoch Times in an email he was “happy to answer any questions you have” but that he needed clearance from the NIAID’s media office. That office then refused to give clearance.
Dr. Jay Bhattacharya, a professor of health policy at Stanford University, said that Dr. Memoli showed bravery when he warned Dr. Fauci against mandates.
“Those mandates have done more to demolish public trust in public health than any single action by public health officials in my professional career, including diminishing public trust in all vaccines.” Dr. Bhattacharya, a frequent critic of the U.S. response to COVID-19, told The Epoch Times via email. “It was risky for Dr. Memoli to speak publicly since he works at the NIH, and the culture of the NIH punishes those who cross powerful scientific bureaucrats like Dr. Fauci or his former boss, Dr. Francis Collins.”
Spread & Containment
Trump “Clearly Hasn’t Learned From His COVID-Era Mistakes”, RFK Jr. Says
Trump "Clearly Hasn’t Learned From His COVID-Era Mistakes", RFK Jr. Says
Authored by Jeff Louderback via The Epoch Times (emphasis ours),
President…

Authored by Jeff Louderback via The Epoch Times (emphasis ours),
President Joe Biden claimed that COVID vaccines are now helping cancer patients during his State of the Union address on March 7, but it was a response on Truth Social from former President Donald Trump that drew the ire of independent presidential candidate Robert F. Kennedy Jr.
During the address, President Biden said: “The pandemic no longer controls our lives. The vaccines that saved us from COVID are now being used to help beat cancer, turning setback into comeback. That’s what America does.”
President Trump wrote: “The Pandemic no longer controls our lives. The VACCINES that saved us from COVID are now being used to help beat cancer—turning setback into comeback. YOU’RE WELCOME JOE. NINE-MONTH APPROVAL TIME VS. 12 YEARS THAT IT WOULD HAVE TAKEN YOU.”
An outspoken critic of President Trump’s COVID response, and the Operation Warp Speed program that escalated the availability of COVID vaccines, Mr. Kennedy said on X, formerly known as Twitter, that “Donald Trump clearly hasn’t learned from his COVID-era mistakes.”
“He fails to recognize how ineffective his warp speed vaccine is as the ninth shot is being recommended to seniors. Even more troubling is the documented harm being caused by the shot to so many innocent children and adults who are suffering myocarditis, pericarditis, and brain inflammation,” Mr. Kennedy remarked.
“This has been confirmed by a CDC-funded study of 99 million people. Instead of bragging about its speedy approval, we should be honestly and transparently debating the abundant evidence that this vaccine may have caused more harm than good.
“I look forward to debating both Trump and Biden on Sept. 16 in San Marcos, Texas.”
Mr. Kennedy announced in April 2023 that he would challenge President Biden for the 2024 Democratic Party presidential nomination before declaring his run as an independent last October, claiming that the Democrat National Committee was “rigging the primary.”
Since the early stages of his campaign, Mr. Kennedy has generated more support than pundits expected from conservatives, moderates, and independents resulting in speculation that he could take votes away from President Trump.
Many Republicans continue to seek a reckoning over the government-imposed pandemic lockdowns and vaccine mandates.
President Trump’s defense of Operation Warp Speed, the program he rolled out in May 2020 to spur the development and distribution of COVID-19 vaccines amid the pandemic, remains a sticking point for some of his supporters.

Operation Warp Speed featured a partnership between the government, the military, and the private sector, with the government paying for millions of vaccine doses to be produced.
President Trump released a statement in March 2021 saying: “I hope everyone remembers when they’re getting the COVID-19 Vaccine, that if I wasn’t President, you wouldn’t be getting that beautiful ‘shot’ for 5 years, at best, and probably wouldn’t be getting it at all. I hope everyone remembers!”
President Trump said about the COVID-19 vaccine in an interview on Fox News in March 2021: “It works incredibly well. Ninety-five percent, maybe even more than that. I would recommend it, and I would recommend it to a lot of people that don’t want to get it and a lot of those people voted for me, frankly.
“But again, we have our freedoms and we have to live by that and I agree with that also. But it’s a great vaccine, it’s a safe vaccine, and it’s something that works.”
On many occasions, President Trump has said that he is not in favor of vaccine mandates.
An environmental attorney, Mr. Kennedy founded Children’s Health Defense, a nonprofit that aims to end childhood health epidemics by promoting vaccine safeguards, among other initiatives.
Last year, Mr. Kennedy told podcaster Joe Rogan that ivermectin was suppressed by the FDA so that the COVID-19 vaccines could be granted emergency use authorization.
He has criticized Big Pharma, vaccine safety, and government mandates for years.
Since launching his presidential campaign, Mr. Kennedy has made his stances on the COVID-19 vaccines, and vaccines in general, a frequent talking point.
“I would argue that the science is very clear right now that they [vaccines] caused a lot more problems than they averted,” Mr. Kennedy said on Piers Morgan Uncensored last April.
“And if you look at the countries that did not vaccinate, they had the lowest death rates, they had the lowest COVID and infection rates.”
Additional data show a “direct correlation” between excess deaths and high vaccination rates in developed countries, he said.
President Trump and Mr. Kennedy have similar views on topics like protecting the U.S.-Mexico border and ending the Russia-Ukraine war.
COVID-19 is the topic where Mr. Kennedy and President Trump seem to differ the most.
Former President Donald Trump intended to “drain the swamp” when he took office in 2017, but he was “intimidated by bureaucrats” at federal agencies and did not accomplish that objective, Mr. Kennedy said on Feb. 5.
Speaking at a voter rally in Tucson, where he collected signatures to get on the Arizona ballot, the independent presidential candidate said President Trump was “earnest” when he vowed to “drain the swamp,” but it was “business as usual” during his term.
John Bolton, who President Trump appointed as a national security adviser, is “the template for a swamp creature,” Mr. Kennedy said.
Scott Gottlieb, who President Trump named to run the FDA, “was Pfizer’s business partner” and eventually returned to Pfizer, Mr. Kennedy said.
Mr. Kennedy said that President Trump had more lobbyists running federal agencies than any president in U.S. history.
“You can’t reform them when you’ve got the swamp creatures running them, and I’m not going to do that. I’m going to do something different,” Mr. Kennedy said.
During the COVID-19 pandemic, President Trump “did not ask the questions that he should have,” he believes.
President Trump “knew that lockdowns were wrong” and then “agreed to lockdowns,” Mr. Kennedy said.
He also “knew that hydroxychloroquine worked, he said it,” Mr. Kennedy explained, adding that he was eventually “rolled over” by Dr. Anthony Fauci and his advisers.

MaryJo Perry, a longtime advocate for vaccine choice and a Trump supporter, thinks votes will be at a premium come Election Day, particularly because the independent and third-party field is becoming more competitive.
Ms. Perry, president of Mississippi Parents for Vaccine Rights, believes advocates for medical freedom could determine who is ultimately president.
She believes that Mr. Kennedy is “pulling votes from Trump” because of the former president’s stance on the vaccines.
“People care about medical freedom. It’s an important issue here in Mississippi, and across the country,” Ms. Perry told The Epoch Times.
“Trump should admit he was wrong about Operation Warp Speed and that COVID vaccines have been dangerous. That would make a difference among people he has offended.”
President Trump won’t lose enough votes to Mr. Kennedy about Operation Warp Speed and COVID vaccines to have a significant impact on the election, Ohio Republican strategist Wes Farno told The Epoch Times.
President Trump won in Ohio by eight percentage points in both 2016 and 2020. The Ohio Republican Party endorsed President Trump for the nomination in 2024.
“The positives of a Trump presidency far outweigh the negatives,” Mr. Farno said. “People are more concerned about their wallet and the economy.
“They are asking themselves if they were better off during President Trump’s term compared to since President Biden took office. The answer to that question is obvious because many Americans are struggling to afford groceries, gas, mortgages, and rent payments.
“America needs President Trump.”
Multiple national polls back Mr. Farno’s view.
As of March 6, the RealClearPolitics average of polls indicates that President Trump has 41.8 percent support in a five-way race that includes President Biden (38.4 percent), Mr. Kennedy (12.7 percent), independent Cornel West (2.6 percent), and Green Party nominee Jill Stein (1.7 percent).
A Pew Research Center study conducted among 10,133 U.S. adults from Feb. 7 to Feb. 11 showed that Democrats and Democrat-leaning independents (42 percent) are more likely than Republicans and GOP-leaning independents (15 percent) to say they have received an updated COVID vaccine.
The poll also reported that just 28 percent of adults say they have received the updated COVID inoculation.
The peer-reviewed multinational study of more than 99 million vaccinated people that Mr. Kennedy referenced in his X post on March 7 was published in the Vaccine journal on Feb. 12.
It aimed to evaluate the risk of 13 adverse events of special interest (AESI) following COVID-19 vaccination. The AESIs spanned three categories—neurological, hematologic (blood), and cardiovascular.
The study reviewed data collected from more than 99 million vaccinated people from eight nations—Argentina, Australia, Canada, Denmark, Finland, France, New Zealand, and Scotland—looking at risks up to 42 days after getting the shots.
Three vaccines—Pfizer and Moderna’s mRNA vaccines as well as AstraZeneca’s viral vector jab—were examined in the study.
Researchers found higher-than-expected cases that they deemed met the threshold to be potential safety signals for multiple AESIs, including for Guillain-Barre syndrome (GBS), cerebral venous sinus thrombosis (CVST), myocarditis, and pericarditis.
A safety signal refers to information that could suggest a potential risk or harm that may be associated with a medical product.
The study identified higher incidences of neurological, cardiovascular, and blood disorder complications than what the researchers expected.
President Trump’s role in Operation Warp Speed, and his continued praise of the COVID vaccine, remains a concern for some voters, including those who still support him.
Krista Cobb is a 40-year-old mother in western Ohio. She voted for President Trump in 2020 and said she would cast her vote for him this November, but she was stunned when she saw his response to President Biden about the COVID-19 vaccine during the State of the Union address.
“I love President Trump and support his policies, but at this point, he has to know they [advisers and health officials] lied about the shot,” Ms. Cobb told The Epoch Times.
“If he continues to promote it, especially after all of the hearings they’ve had about it in Congress, the side effects, and cover-ups on Capitol Hill, at what point does he become the same as the people who have lied?” Ms. Cobb added.
“I think he should distance himself from talk about Operation Warp Speed and even admit that he was wrong—that the vaccines have not had the impact he was told they would have. If he did that, people would respect him even more.”
International
The next pandemic? It’s already here for Earth’s wildlife
Bird flu is decimating species already threatened by climate change and habitat loss.

I am a conservation biologist who studies emerging infectious diseases. When people ask me what I think the next pandemic will be I often say that we are in the midst of one – it’s just afflicting a great many species more than ours.
I am referring to the highly pathogenic strain of avian influenza H5N1 (HPAI H5N1), otherwise known as bird flu, which has killed millions of birds and unknown numbers of mammals, particularly during the past three years.
This is the strain that emerged in domestic geese in China in 1997 and quickly jumped to humans in south-east Asia with a mortality rate of around 40-50%. My research group encountered the virus when it killed a mammal, an endangered Owston’s palm civet, in a captive breeding programme in Cuc Phuong National Park Vietnam in 2005.
How these animals caught bird flu was never confirmed. Their diet is mainly earthworms, so they had not been infected by eating diseased poultry like many captive tigers in the region.
This discovery prompted us to collate all confirmed reports of fatal infection with bird flu to assess just how broad a threat to wildlife this virus might pose.
This is how a newly discovered virus in Chinese poultry came to threaten so much of the world’s biodiversity.
The first signs
Until December 2005, most confirmed infections had been found in a few zoos and rescue centres in Thailand and Cambodia. Our analysis in 2006 showed that nearly half (48%) of all the different groups of birds (known to taxonomists as “orders”) contained a species in which a fatal infection of bird flu had been reported. These 13 orders comprised 84% of all bird species.
We reasoned 20 years ago that the strains of H5N1 circulating were probably highly pathogenic to all bird orders. We also showed that the list of confirmed infected species included those that were globally threatened and that important habitats, such as Vietnam’s Mekong delta, lay close to reported poultry outbreaks.
Mammals known to be susceptible to bird flu during the early 2000s included primates, rodents, pigs and rabbits. Large carnivores such as Bengal tigers and clouded leopards were reported to have been killed, as well as domestic cats.
Our 2006 paper showed the ease with which this virus crossed species barriers and suggested it might one day produce a pandemic-scale threat to global biodiversity.
Unfortunately, our warnings were correct.
A roving sickness
Two decades on, bird flu is killing species from the high Arctic to mainland Antarctica.
In the past couple of years, bird flu has spread rapidly across Europe and infiltrated North and South America, killing millions of poultry and a variety of bird and mammal species. A recent paper found that 26 countries have reported at least 48 mammal species that have died from the virus since 2020, when the latest increase in reported infections started.
Not even the ocean is safe. Since 2020, 13 species of aquatic mammal have succumbed, including American sea lions, porpoises and dolphins, often dying in their thousands in South America. A wide range of scavenging and predatory mammals that live on land are now also confirmed to be susceptible, including mountain lions, lynx, brown, black and polar bears.
The UK alone has lost over 75% of its great skuas and seen a 25% decline in northern gannets. Recent declines in sandwich terns (35%) and common terns (42%) were also largely driven by the virus.
Scientists haven’t managed to completely sequence the virus in all affected species. Research and continuous surveillance could tell us how adaptable it ultimately becomes, and whether it can jump to even more species. We know it can already infect humans – one or more genetic mutations may make it more infectious.
At the crossroads
Between January 1 2003 and December 21 2023, 882 cases of human infection with the H5N1 virus were reported from 23 countries, of which 461 (52%) were fatal.
Of these fatal cases, more than half were in Vietnam, China, Cambodia and Laos. Poultry-to-human infections were first recorded in Cambodia in December 2003. Intermittent cases were reported until 2014, followed by a gap until 2023, yielding 41 deaths from 64 cases. The subtype of H5N1 virus responsible has been detected in poultry in Cambodia since 2014. In the early 2000s, the H5N1 virus circulating had a high human mortality rate, so it is worrying that we are now starting to see people dying after contact with poultry again.
It’s not just H5 subtypes of bird flu that concern humans. The H10N1 virus was originally isolated from wild birds in South Korea, but has also been reported in samples from China and Mongolia.
Recent research found that these particular virus subtypes may be able to jump to humans after they were found to be pathogenic in laboratory mice and ferrets. The first person who was confirmed to be infected with H10N5 died in China on January 27 2024, but this patient was also suffering from seasonal flu (H3N2). They had been exposed to live poultry which also tested positive for H10N5.
Species already threatened with extinction are among those which have died due to bird flu in the past three years. The first deaths from the virus in mainland Antarctica have just been confirmed in skuas, highlighting a looming threat to penguin colonies whose eggs and chicks skuas prey on. Humboldt penguins have already been killed by the virus in Chile.

How can we stem this tsunami of H5N1 and other avian influenzas? Completely overhaul poultry production on a global scale. Make farms self-sufficient in rearing eggs and chicks instead of exporting them internationally. The trend towards megafarms containing over a million birds must be stopped in its tracks.
To prevent the worst outcomes for this virus, we must revisit its primary source: the incubator of intensive poultry farms.
Diana Bell does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
genetic pandemic mortality spread deaths south korea south america europe uk china-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International4 days ago
International4 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















