Government
Pick Up the Pieces: Invivyd Engineers New COVID-19 Monoclonal Candidate
In September, Invivyd dosed its first patient in the Phase III CANOPY pivotal trial (NCT06039449), designed to assess VYD222’s ability to protect against…

By Alex Philippidis
Nearly two years after the Omicron variant bested the lead candidate of its predecessor company, Adagio—causing its stock to plunge 79%, its founding CEO to resign, and the company to rebrand—Invivyd has picked up the pieces with a new pipeline anchor, a monoclonal antibody (mAb) for preventing COVID-19 derived from the one that failed.
VYD222 is a re-engineered mAb that differs from the failed ADG20, now called adintrevimab, by eight different amino acids. That allows VYD222 to shift slightly where it binds with the spike protein of SARS-CoV-2—a change that Invivyd reasons will make the antibody much better able than adintrevimab at preventing symptomatic COVID-19 as the virus rapidly evolves, forming new variants.
The re-engineering that wrought VYD222 reflects a change in strategy since Adagio became Invivyd, toward developing a continuous repertoire of SARS-CoV-2 neutralizing mAbs.
“We’ve really shifted this thesis from a singular antibody that would be designed, developed, and assumed to have ongoing activity, to one where we seek to meet viral evolution and the speed of viral evolution that we’ve seen in SARS-CoV-2,” Invivyd CEO Dave Hering told GEN Edge. “We need to do this continuously. We need to be able to provide antibodies on a regular updated basis that meet the new variants.”
In September, Invivyd dosed its first patient in the Phase III CANOPY pivotal trial (NCT06039449), designed to assess VYD222’s ability to protect against symptomatic COVID-19 at a 4,500 mg dose.
The trial will evaluate some 750 participants in two cohorts: One of about 300 significantly immunocompromised participants, the other about 450 participants at risk of exposure to SARS-CoV-2, randomized 2:1 to VYD222 or placebo. The primary endpoint for Cohort A is serum neutralizing titers against relevant SARS-CoV-2 variants at Day 28. For Cohort B, the primary endpoint is safety and tolerability.
Investors responded with a mini-surge that sent Invivyd’s stock price up 7%, from $1.77 a day before the announcement to $1.89 a day after. Since then, the stock has fallen 20%, closing at $1.51 on Monday.
However, Michael J. Yee, equity analyst with Jefferies, took a more positive view of the company over the summer, giving Invivyd high marks for its pivotal study design.
“We think the Phase III design is smart and aligned with FDA, which will allow them to quickly enroll the needed [patients] for the minimally sufficient 28 days titer level data and ‘all-comer’ safety data, while leveraging clinical data from the previous adintrevimab as a prototype drug, to put together a comprehensive data package for a potential EUA in a timely fashion,” Yee wrote in August.
Potential EUA filing
Initial primary endpoint data is expected to be released by the end of 2023 or early in the first quarter of 2024. Invivyd hopes that data will be positive enough to support a potential emergency use authorization (EUA) filing for VYD222, based on what the company calls its “general alignment” with an FDA regulatory pathway for that and anticipated follow-on mAb candidates.
Invivyd is not disclosing how many patients have since joined the initial participant in CANOPY, though Hering said the trial is going as planned, and is encouraged by what he called “a fair amount of outreach” to the company’s investor relations from patients interested in joining the study.
“Interestingly, even people not immunocompromised have been quite interested in enrolling in the trial. I attribute that to, among other things, the fact that people are still seeing COVID-19 cases in their communities and in their circles,” Hering said.
Over the summer, Invivyd said VYD222 generated positive initial data in its ongoing Phase I trial (NCT05791318) showing that a single administration of VYD222 was generally well-tolerated at all three dose levels tested with no serious adverse events. All dose levels tested showed robust neutralization activity against Omicron XBB.1.5 at Day 7.
“Given the dose-dependent strong 7-day titer data shown in Phase I earlier, we think the Phase III could easily replicate Phase I with stronger powering testing only the highest Phase I IV [intravenous] dose of 4500 mg,” Yee observed. “The titer levels may come down a bit going from Day 7 to Day 28, but the 4500 mg dose showed huge titer levels up to 16,800 or a ~121-fold increase in titer level vs baseline in Phase I which leaves enough ‘buffer’ and we should still see a strong effect at this readout.”
“In an optimal scenario, IVVD could be launching under EUA for immuno-compromised [patients] in the label—and have revenue come in as soon as the second half of 2024, Yee predicted, while maintaining his firm’s “Hold” rating and 12-month price target of $1.50.
Multi-billion-dollar market
If that happens, he added, VYD222 could share a multi-billion dollar market for COVID-19-preventing antibodies with AstraZeneca, which is studying its next-generation long-acting antibody (LAAB) AZD3152 in the Phase II/III SUPERNOVA trial (NCT05648110).
Derived from B-cells donated by convalescent patients after SARS-CoV-2 infection, AZD3152 is optimized with a half-life extension and reduced Fc effector function and complement C1q binding platform expected to confer protection from COVID-19 for six months. A similar platform was used by Evusheld (tixagevimab and cilgavimab), AstraZeneca’s COVID-19 antibody combination which gained EUA last year, then lost authorization in January due to lack of effectiveness against newer variants. AstraZeneca has said it could make AZD3153 available by year’s end via EUA, if data from the trial proves positive.
Hering said the market for VYD222 is large enough, depending on how immunocompromised is quantified and classified, to range from 8 to 18 million people. “We started, as you can imagine, doing work to look into the different populations that make up immunocompromised organ transplant patients: People with different oncology and cancers, in particular blood cancers. People on immune suppressants for rheumatoid arthritis, or multiple sclerosis dialysis,” Hering said.
Although not technically considered immunocompromised, he noted, older adults develop problems with their immune systems: “You could also see in some of those populations a need for additional products beyond vaccines.”
“There’s a huge unmet need in particular, in these populations we’ve been talking about, and a real need to supplement the medicine cabinet with products like ours,” Hering said. “So, we’ve been working with regulators on how to do this efficiently and how to do this on an ongoing basis, really, with that goal in mind.”
Invivyd was launched as Adagio Therapeutics, a spinout of Adimab, at the height of the COVID-19 pandemic in 2020. After raising $130 million in two financing rounds, Adagio initially sought to develop ADG2, an engineered antibody that successfully offered complete protection against severe SARS-CoV-2 and SARS-CoV in mouse models.
By December 2020, Adagio had shifted its focus to adintrevimab, a newer version of ADG2 that was engineered to offer a longer duration of protection by extending the antibody’s serum half-life.
The new antibody, then called ADG20, showed promise against not only COVID-19 but also other coronaviruses, including SARS-CoV-1 (SARS-CoV) and MERS. “Our view was, it’s great to have a treatment for this particular outbreak, but why not aim a little bit higher and figure out a way of finding antibodies that neutralize the entire clade of coronaviruses that target human ACE2?” Adagio co-founder and CEO Tillman Gerngross, PhD, told GEN Edge in 2020, referring to angiotensin-converting enzyme 2.
By February 2021, Adagio took adintrevimab into the clinic, dosing the first patient in a Phase I trial, and rapidly advancing the mAb into two late-phase studies, the Phase III EVADE trial (NCT04859517) assessing the antibody’s prevention of COVID-19; and the Phase II/III STAMP trial (NCT04805671), evaluating ADG20 as a treatment for COVID-19.
Hitting turbulence
Adagio hit turbulence in December 2021, when the company acknowledged that ADG20 had failed to effectively neutralize the Omicron variant of SARS-CoV-2 (B1.1.529) in lab testing as the company had publicly predicted a month earlier. After results failed to fulfill Adagio’s rosy prediction, which had sent shares near doubling, investors punished the company severely, sending its shares cratering 79% in a single day to $7.26 from $34.26.
Two months later in February 2022, Gerngross resigned. He was succeeded by Hering, who had joined Adagio seven months earlier as COO. In September 2022, Adagio rebranded as Invivyd, a name intended to reflect the company’s business focus of applying its discovery platform to develop and commercialize antibodies that transcend the limits of the human immune system to better prevent and treat infectious respiratory viral diseases, starting with COVID-19.
Invivyd re-engineered adintrevimab into VYD222 using its integrated discovery platform:
- First, monitor viral evolution and map common mutational escape routes to predict future variants of concern.
- Use deep B-cell mining capabilities licensed from Adimab to isolate broadly neutralizing antibodies that target rare viral epitopes that are not under strong immune pressure, thus increasing the probability of sustained utility.
- Engineer antibodies to improve their potency, breadth, biophysical properties, and developability. Using affinity maturation, Invivyd can engineer candidates to have high activity against specific viral variants it identifies as potential future variants of concern.
“When the omicron mutations occurred and reduced activity, this engineering re-established those bindings and recreated the activity and the binding. That has been really useful for a couple of reasons,” Hering said.
First, the re-engineering allowed Invivyd to apply a new regulatory framework for its Phase III trial allowing “immunobridging” through the use of data from serum-neutralizing titers as well as previously generated clinical trial data from a prototype mAb—in this case, adintrevimab (ADG20).
Second, adintrevimab met the FDA’s three criteria for a prototype mAb—it was initially established as a mAb; it completed pivotal, double-blind clinical trials; and it uses the same manufacturing platform and has activity against new current circulating variants, which VYD222 has shown. Adintrevimab remains within Invivyd’s pipeline, with trials having concluded and a package of data ready to submit for an EUA filing dependent on variant susceptibility: “If the virus mutates in a way that generates activity for ADG20, we still have inventory of the product.”
Adintrevimab and VYD222 are the only two named candidates listed in Invivyd’s pipeline. The company has three other COVID-19 mAb candidates, identified only as #3, #4, and #5, in discovery and preclinical phases—plus a mAb combination candidate for prevention of influenza, in the early discovery phase.
As new variants emerge, Hering said, Invivyd envisions developing several new antibodies in response. “Our approach is to look at a set of, basically, a family of different antibodies. VYD222 comes from this ADG20 lineage. We do see that ‘222 can be re-engineered further,” Hering said.
Zigging and zagging
“We’ve done some of that work already looking at it versus the current variants now, but we’re starting to look at this as, maybe we have a handful of families of model antibodies that are on different parts of the spike, protein, different areas of the receptor binding domain, non-overlapping epitopes that would give us coverage,” Hering explained. “So, if the virus zigs, we can zag. If it zags, we can zig.”
Hering said the annual model for availability of COVID-19 and flu vaccines could serve as a similar model for generating antibodies that keep pace with newer variants and any older ones that may re-emerge.
“We’re talking with regulators now about how that could work, but really thinking through that again, on a yearly basis, we would provide updated antibodies that work against that year’s variants,” he added.
Since COVID-19 emerged in late 2019, the FDA has granted EUAs to 13 treatments, only to withdraw five of those approvals as the treatments proved unable to combat newer variants of SARS-CoV-2. The withdrawn treatments include Regeneron Pharmaceuticals’ REGEN-COV combination (casirivimab and Imdevimab), Eli Lilly’s combination bamlanivimab and etesevimab, GlaxoSmithKline (GSK)’s sotrovimab, AstraZeneca’s Evusheld, and Lilly’s bebtelovimab.
Of the COVID-19 mAbs on the market, the bestseller by far is Pfizer’s Paxlovid®, which generated $4.4 billion in revenues in the first nine months of this year. But that’s down 74% from the $17 billion Pfizer racked up during January-September 2022.
The mAb most recently granted an EUA for COVID-19 covers a niche population. InflaRx’s Gohibic (vilobelimab) was authorized in April to treat COVID-19 in hospitalized adults when initiated within 48 hours of receiving invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO).
Invivyd’s headcount now stands at about 80–90 people, Hering said, in line with the 84 full-time employees reported as of February 1 but below the 101 disclosed a year earlier, according to regulatory filings. However, Invivyd plans to grow its staff further should it need additional people to commercialize VYD222.
“That’s still in the planning stages and we’re looking to see how that all unfolds,” Hering cautioned. “Certainly, we’re starting to build inventory to be ready for a launch, doing all of the different market access and different components that you would expect so that we could be ready for potential launch.”
Does Invivyd envision standing alone in infectious disease, or evolving into the infectious disease subsidiary of some biopharma giant?
“Too early to tell right now,” Hering said. “We’re very excited for what we’ve accomplished so far, and we’ll certainly look at that when and if that situation occurs. But right now, we’re focused on VYD222 and getting ready to potentially launch it.”
“Our belief is that COVID-19, unfortunately, is here to stay. And year after year people are going to need products like ours—hopefully, fingers crossed—to provide protection and prevention on an ongoing basis,” Hering added.
Alex Philippidis is senior business editor of GEN.
The post Pick Up the Pieces: Invivyd Engineers New COVID-19 Monoclonal Candidate appeared first on GEN - Genetic Engineering and Biotechnology News.
emergency use authorization pandemic covid-19 treatment testing fda clinical trials preclinical genetic antibodiesInternational
Four Years Ago This Week, Freedom Was Torched
Four Years Ago This Week, Freedom Was Torched
Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare…

Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare quotes the soothsayer’s warning Julius Caesar about what turned out to be an impending assassination on March 15. The death of American liberty happened around the same time four years ago, when the orders went out from all levels of government to close all indoor and outdoor venues where people gather.
It was not quite a law and it was never voted on by anyone. Seemingly out of nowhere, people who the public had largely ignored, the public health bureaucrats, all united to tell the executives in charge – mayors, governors, and the president – that the only way to deal with a respiratory virus was to scrap freedom and the Bill of Rights.
And they did, not only in the US but all over the world.
The forced closures in the US began on March 6 when the mayor of Austin, Texas, announced the shutdown of the technology and arts festival South by Southwest. Hundreds of thousands of contracts, of attendees and vendors, were instantly scrapped. The mayor said he was acting on the advice of his health experts and they in turn pointed to the CDC, which in turn pointed to the World Health Organization, which in turn pointed to member states and so on.
There was no record of Covid in Austin, Texas, that day but they were sure they were doing their part to stop the spread. It was the first deployment of the “Zero Covid” strategy that became, for a time, official US policy, just as in China.
It was never clear precisely who to blame or who would take responsibility, legal or otherwise.
This Friday evening press conference in Austin was just the beginning. By the next Thursday evening, the lockdown mania reached a full crescendo. Donald Trump went on nationwide television to announce that everything was under control but that he was stopping all travel in and out of US borders, from Europe, the UK, Australia, and New Zealand. American citizens would need to return by Monday or be stuck.
Americans abroad panicked while spending on tickets home and crowded into international airports with waits up to 8 hours standing shoulder to shoulder. It was the first clear sign: there would be no consistency in the deployment of these edicts.
There is no historical record of any American president ever issuing global travel restrictions like this without a declaration of war. Until then, and since the age of travel began, every American had taken it for granted that he could buy a ticket and board a plane. That was no longer possible. Very quickly it became even difficult to travel state to state, as most states eventually implemented a two-week quarantine rule.
The next day, Friday March 13, Broadway closed and New York City began to empty out as any residents who could went to summer homes or out of state.
On that day, the Trump administration declared the national emergency by invoking the Stafford Act which triggers new powers and resources to the Federal Emergency Management Administration.
In addition, the Department of Health and Human Services issued a classified document, only to be released to the public months later. The document initiated the lockdowns. It still does not exist on any government website.
The White House Coronavirus Response Task Force, led by the Vice President, will coordinate a whole-of-government approach, including governors, state and local officials, and members of Congress, to develop the best options for the safety, well-being, and health of the American people. HHS is the LFA [Lead Federal Agency] for coordinating the federal response to COVID-19.
Closures were guaranteed:
Recommend significantly limiting public gatherings and cancellation of almost all sporting events, performances, and public and private meetings that cannot be convened by phone. Consider school closures. Issue widespread ‘stay at home’ directives for public and private organizations, with nearly 100% telework for some, although critical public services and infrastructure may need to retain skeleton crews. Law enforcement could shift to focus more on crime prevention, as routine monitoring of storefronts could be important.
In this vision of turnkey totalitarian control of society, the vaccine was pre-approved: “Partner with pharmaceutical industry to produce anti-virals and vaccine.”
The National Security Council was put in charge of policy making. The CDC was just the marketing operation. That’s why it felt like martial law. Without using those words, that’s what was being declared. It even urged information management, with censorship strongly implied.
The timing here is fascinating. This document came out on a Friday. But according to every autobiographical account – from Mike Pence and Scott Gottlieb to Deborah Birx and Jared Kushner – the gathered team did not meet with Trump himself until the weekend of the 14th and 15th, Saturday and Sunday.
According to their account, this was his first real encounter with the urge that he lock down the whole country. He reluctantly agreed to 15 days to flatten the curve. He announced this on Monday the 16th with the famous line: “All public and private venues where people gather should be closed.”
This makes no sense. The decision had already been made and all enabling documents were already in circulation.
There are only two possibilities.
One: the Department of Homeland Security issued this March 13 HHS document without Trump’s knowledge or authority. That seems unlikely.
Two: Kushner, Birx, Pence, and Gottlieb are lying. They decided on a story and they are sticking to it.
Trump himself has never explained the timeline or precisely when he decided to greenlight the lockdowns. To this day, he avoids the issue beyond his constant claim that he doesn’t get enough credit for his handling of the pandemic.
With Nixon, the famous question was always what did he know and when did he know it? When it comes to Trump and insofar as concerns Covid lockdowns – unlike the fake allegations of collusion with Russia – we have no investigations. To this day, no one in the corporate media seems even slightly interested in why, how, or when human rights got abolished by bureaucratic edict.
As part of the lockdowns, the Cybersecurity and Infrastructure Security Agency, which was and is part of the Department of Homeland Security, as set up in 2018, broke the entire American labor force into essential and nonessential.
They also set up and enforced censorship protocols, which is why it seemed like so few objected. In addition, CISA was tasked with overseeing mail-in ballots.
Only 8 days into the 15, Trump announced that he wanted to open the country by Easter, which was on April 12. His announcement on March 24 was treated as outrageous and irresponsible by the national press but keep in mind: Easter would already take us beyond the initial two-week lockdown. What seemed to be an opening was an extension of closing.
This announcement by Trump encouraged Birx and Fauci to ask for an additional 30 days of lockdown, which Trump granted. Even on April 23, Trump told Georgia and Florida, which had made noises about reopening, that “It’s too soon.” He publicly fought with the governor of Georgia, who was first to open his state.
Before the 15 days was over, Congress passed and the president signed the 880-page CARES Act, which authorized the distribution of $2 trillion to states, businesses, and individuals, thus guaranteeing that lockdowns would continue for the duration.
There was never a stated exit plan beyond Birx’s public statements that she wanted zero cases of Covid in the country. That was never going to happen. It is very likely that the virus had already been circulating in the US and Canada from October 2019. A famous seroprevalence study by Jay Bhattacharya came out in May 2020 discerning that infections and immunity were already widespread in the California county they examined.
What that implied was two crucial points: there was zero hope for the Zero Covid mission and this pandemic would end as they all did, through endemicity via exposure, not from a vaccine as such. That was certainly not the message that was being broadcast from Washington. The growing sense at the time was that we all had to sit tight and just wait for the inoculation on which pharmaceutical companies were working.
By summer 2020, you recall what happened. A restless generation of kids fed up with this stay-at-home nonsense seized on the opportunity to protest racial injustice in the killing of George Floyd. Public health officials approved of these gatherings – unlike protests against lockdowns – on grounds that racism was a virus even more serious than Covid. Some of these protests got out of hand and became violent and destructive.
Meanwhile, substance abuse rage – the liquor and weed stores never closed – and immune systems were being degraded by lack of normal exposure, exactly as the Bakersfield doctors had predicted. Millions of small businesses had closed. The learning losses from school closures were mounting, as it turned out that Zoom school was near worthless.
It was about this time that Trump seemed to figure out – thanks to the wise council of Dr. Scott Atlas – that he had been played and started urging states to reopen. But it was strange: he seemed to be less in the position of being a president in charge and more of a public pundit, Tweeting out his wishes until his account was banned. He was unable to put the worms back in the can that he had approved opening.
By that time, and by all accounts, Trump was convinced that the whole effort was a mistake, that he had been trolled into wrecking the country he promised to make great. It was too late. Mail-in ballots had been widely approved, the country was in shambles, the media and public health bureaucrats were ruling the airwaves, and his final months of the campaign failed even to come to grips with the reality on the ground.
At the time, many people had predicted that once Biden took office and the vaccine was released, Covid would be declared to have been beaten. But that didn’t happen and mainly for one reason: resistance to the vaccine was more intense than anyone had predicted. The Biden administration attempted to impose mandates on the entire US workforce. Thanks to a Supreme Court ruling, that effort was thwarted but not before HR departments around the country had already implemented them.
As the months rolled on – and four major cities closed all public accommodations to the unvaccinated, who were being demonized for prolonging the pandemic – it became clear that the vaccine could not and would not stop infection or transmission, which means that this shot could not be classified as a public health benefit. Even as a private benefit, the evidence was mixed. Any protection it provided was short-lived and reports of vaccine injury began to mount. Even now, we cannot gain full clarity on the scale of the problem because essential data and documentation remains classified.
After four years, we find ourselves in a strange position. We still do not know precisely what unfolded in mid-March 2020: who made what decisions, when, and why. There has been no serious attempt at any high level to provide a clear accounting much less assign blame.
Not even Tucker Carlson, who reportedly played a crucial role in getting Trump to panic over the virus, will tell us the source of his own information or what his source told him. There have been a series of valuable hearings in the House and Senate but they have received little to no press attention, and none have focus on the lockdown orders themselves.
The prevailing attitude in public life is just to forget the whole thing. And yet we live now in a country very different from the one we inhabited five years ago. Our media is captured. Social media is widely censored in violation of the First Amendment, a problem being taken up by the Supreme Court this month with no certainty of the outcome. The administrative state that seized control has not given up power. Crime has been normalized. Art and music institutions are on the rocks. Public trust in all official institutions is at rock bottom. We don’t even know if we can trust the elections anymore.
In the early days of lockdown, Henry Kissinger warned that if the mitigation plan does not go well, the world will find itself set “on fire.” He died in 2023. Meanwhile, the world is indeed on fire. The essential struggle in every country on earth today concerns the battle between the authority and power of permanent administration apparatus of the state – the very one that took total control in lockdowns – and the enlightenment ideal of a government that is responsible to the will of the people and the moral demand for freedom and rights.
How this struggle turns out is the essential story of our times.
CODA: I’m embedding a copy of PanCAP Adapted, as annotated by Debbie Lerman. You might need to download the whole thing to see the annotations. If you can help with research, please do.
* * *
Jeffrey Tucker is the author of the excellent new book 'Life After Lock-Down'
Government
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
Authored by Jack Phillips via The Epoch Times (emphasis ours),
A…

Authored by Jack Phillips via The Epoch Times (emphasis ours),
A U.S. Centers for Disease Control (CDC) paper released Thursday found that thousands of young children have been taken to the emergency room over the past several years after taking the very common sleep-aid supplement melatonin.
The agency said that melatonin, which can come in gummies that are meant for adults, was implicated in about 7 percent of all emergency room visits for young children and infants “for unsupervised medication ingestions,” adding that many incidents were linked to the ingestion of gummy formulations that were flavored. Those incidents occurred between the years 2019 and 2022.
Melatonin is a hormone produced by the human body to regulate its sleep cycle. Supplements, which are sold in a number of different formulas, are generally taken before falling asleep and are popular among people suffering from insomnia, jet lag, chronic pain, or other problems.
The supplement isn’t regulated by the U.S. Food and Drug Administration and does not require child-resistant packaging. However, a number of supplement companies include caps or lids that are difficult for children to open.
The CDC report said that a significant number of melatonin-ingestion cases among young children were due to the children opening bottles that had not been properly closed or were within their reach. Thursday’s report, the agency said, “highlights the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight,” including melatonin.
The approximately 11,000 emergency department visits for unsupervised melatonin ingestions by infants and young children during 2019–2022 highlight the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight.
The CDC notes that melatonin use among Americans has increased five-fold over the past 25 years or so. That has coincided with a 530 percent increase in poison center calls for melatonin exposures to children between 2012 and 2021, it said, as well as a 420 percent increase in emergency visits for unsupervised melatonin ingestion by young children or infants between 2009 and 2020.
Some health officials advise that children under the age of 3 should avoid taking melatonin unless a doctor says otherwise. Side effects include drowsiness, headaches, agitation, dizziness, and bed wetting.
Other symptoms of too much melatonin include nausea, diarrhea, joint pain, anxiety, and irritability. The supplement can also impact blood pressure.
However, there is no established threshold for a melatonin overdose, officials have said. Most adult melatonin supplements contain a maximum of 10 milligrams of melatonin per serving, and some contain less.
Many people can tolerate even relatively large doses of melatonin without significant harm, officials say. But there is no antidote for an overdose. In cases of a child accidentally ingesting melatonin, doctors often ask a reliable adult to monitor them at home.
Dr. Cora Collette Breuner, with the Seattle Children’s Hospital at the University of Washington, told CNN that parents should speak with a doctor before giving their children the supplement.
“I also tell families, this is not something your child should take forever. Nobody knows what the long-term effects of taking this is on your child’s growth and development,” she told the outlet. “Taking away blue-light-emitting smartphones, tablets, laptops, and television at least two hours before bed will keep melatonin production humming along, as will reading or listening to bedtime stories in a softly lit room, taking a warm bath, or doing light stretches.”
In 2022, researchers found that in 2021, U.S. poison control centers received more than 52,000 calls about children consuming worrisome amounts of the dietary supplement. That’s a six-fold increase from about a decade earlier. Most such calls are about young children who accidentally got into bottles of melatonin, some of which come in the form of gummies for kids, the report said.
Dr. Karima Lelak, an emergency physician at Children’s Hospital of Michigan and the lead author of the study published in 2022 by the CDC, found that in about 83 percent of those calls, the children did not show any symptoms.
However, other children had vomiting, altered breathing, or other symptoms. Over the 10 years studied, more than 4,000 children were hospitalized, five were put on machines to help them breathe, and two children under the age of two died. Most of the hospitalized children were teenagers, and many of those ingestions were thought to be suicide attempts.
Those researchers also suggested that COVID-19 lockdowns and virtual learning forced more children to be at home all day, meaning there were more opportunities for kids to access melatonin. Also, those restrictions may have caused sleep-disrupting stress and anxiety, leading more families to consider melatonin, they suggested.
The Associated Press contributed to this report.
International
Red Candle In The Wind
Red Candle In The Wind
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by…
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by printing at 275,000 against a consensus call of 200,000. We say superficially, because the downward revisions to prior months totalled 167,000 for December and January, taking the total change in employed persons well below the implied forecast, and helping the unemployment rate to pop two-ticks to 3.9%. The U6 underemployment rate also rose from 7.2% to 7.3%, while average hourly earnings growth fell to 0.2% m-o-m and average weekly hours worked languished at 34.3, equalling pre-pandemic lows.
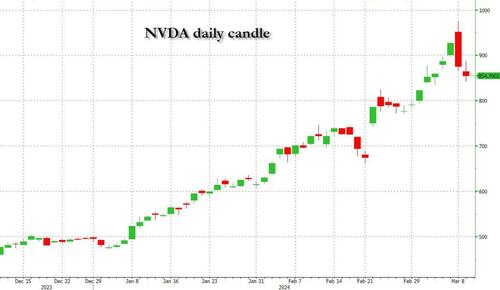
Undeterred by the devil in the detail, the algos sprang into action once exchanges opened. Market darling NVIDIA hit a new intraday high of $974 before (presumably) the humans took over and sold the stock down more than 10% to close at $875.28. If our suspicions are correct that it was the AIs buying before the humans started selling (no doubt triggering trailing stops on the way down), the irony is not lost on us.
The 1-day chart for NVIDIA now makes for interesting viewing, because the red candle posted on Friday presents quite a strong bearish engulfing signal. Volume traded on the day was almost double the 15-day simple moving average, and similar price action is observable on the 1-day charts for both Intel and AMD. Regular readers will be aware that we have expressed incredulity in the past about the durability the AI thematic melt-up, so it will be interesting to see whether Friday’s sell off is just a profit-taking blip, or a genuine trend reversal.
AI equities aside, this week ought to be important for markets because the BTFP program expires today. That means that the Fed will no longer be loaning cash to the banking system in exchange for collateral pledged at-par. The KBW Regional Banking index has so far taken this in its stride and is trading 30% above the lows established during the mini banking crisis of this time last year, but the Fed’s liquidity facility was effectively an exercise in can-kicking that makes regional banks a sector of the market worth paying attention to in the weeks ahead. Even here in Sydney, regulators are warning of external risks posed to the banking sector from scheduled refinancing of commercial real estate loans following sharp falls in valuations.
Markets are sending signals in other sectors, too. Gold closed at a new record-high of $2178/oz on Friday after trading above $2200/oz briefly. Gold has been going ballistic since the Friday before last, posting gains even on days where 2-year Treasury yields have risen. Gold bugs are buying as real yields fall from the October highs and inflation breakevens creep higher. This is particularly interesting as gold ETFs have been recording net outflows; suggesting that price gains aren’t being driven by a retail pile-in. Are gold buyers now betting on a stagflationary outcome where the Fed cuts without inflation being anchored at the 2% target? The price action around the US CPI release tomorrow ought to be illuminating.
Leaving the day-to-day movements to one side, we are also seeing further signs of structural change at the macro level. The UK budget last week included a provision for the creation of a British ISA. That is, an Individual Savings Account that provides tax breaks to savers who invest their money in the stock of British companies. This follows moves last year to encourage pension funds to head up the risk curve by allocating 5% of their capital to unlisted investments.
As a Hail Mary option for a government cruising toward an electoral drubbing it’s a curious choice, but it’s worth highlighting as cash-strapped governments increasingly see private savings pools as a funding solution for their spending priorities.
Of course, the UK is not alone in making creeping moves towards financial repression. In contrast to announcements today of increased trade liberalisation, Australian Treasurer Jim Chalmers has in the recent past flagged his interest in tapping private pension savings to fund state spending priorities, including defence, public housing and renewable energy projects. Both the UK and Australia appear intent on finding ways to open up the lungs of their economies, but government wants more say in directing private capital flows for state goals.
So, how far is the blurring of the lines between free markets and state planning likely to go? Given the immense and varied budgetary (and security) pressures that governments are facing, could we see a re-up of WWII-era Victory bonds, where private investors are encouraged to do their patriotic duty by directly financing government at negative real rates?
That would really light a fire under the gold market.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International4 days ago
International4 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges






















