Government
Pfizer 2020: Cancer and COVID-19
Pfizer 2020: Cancer and COVID-19

Pfizer advanced the cancer pipeline in 2019, but with the advent of the COVID-19 pandemic, the company threw its scientific strength at generating a vaccine against the novel coronovirus.
By Christiane Truelove • chris.truelove@medadnews.com

Pfizer Inc.
235 East 42nd Street
New York, NY 10017
Phone: 212-733-2323
Website: pfizer.com
FINANCIAL PERFORMANCE
(All figures are in millions of dollars, except EPS)
2019
Revenue $51,750
Net income $16,273
Diluted EPS $2.87
R&D expense $8,650
1H 2020
Revenue $23,829
Net income $6,828
Diluted EPS $1.22
R&D expense $3,856
BEST-SELLING Rx PRODUCTS
(All sales are in millions of dollars)
2019
Prevnar 13/Prevenar 13 $5,847
Ibrance $4,961
Eliquis* $4,220
Lyrica $3,321
Xeljanz $2,242
Lipitor $1,973
Enbrel $1,699
Chantix/ Champix $1,107
Norvasc $950
Sutent $936
Xtandi ** $838
Premarin family $734
Celebrex $719
Sulperazon $684
Inflectra/Remsima $625
Xalkori $530
1H 2020
Ibrance $2,598
Eliquis* $2,572
Prevnar 13/Prevenar 13 $2,566
Xeljanz $1,086
Lipitor $836
Lyrica $706
Enbrel $684
Vyndaqel/Vyndamax $508
Chantix/Champix $505
Xtandi** $475
Norvasc $419
Sutent $414
Inlyta $364
Inflectra/Remsima $308
Premarin family $304
Celebrex $295
Sulperazon $289
Xalkori $287
Notes:
* – Includes alliance revenue and direct sales
** – Includes alliance revenue
Outcomes Creativity Index Score: 23
Manny Awards – N/A
Cannes Lions – N/A
LIA: Health & Wellness – 4
Clio Health – 8
One Show: HW&P – N/A
MM&M Awards – 3
Global Awards – 8
Creative Floor Awards – N/A
For Pfizer, 2019 was a busy year, according to Dr. Albert Bourla, Pfizer’s chairman and CEO. The year was highlighted by solid financial performance, shareholder-friendly capital allocation, the strengthening of the company’s pipeline, as well as the formation of the consumer healthcare joint venture with GlaxoSmithKline. Pfizer also announced a definitive agreement to combine its Upjohn unit with Mylan to create a new global pharmaceutical company, Viatris, which Dr. Bourla says marks an important milestone in Pfizer’s evolution toward becoming a more focused, global leader in innovative medicines.

“Pfizer calls on all members of the innovation ecosystem – from large pharmaceutical companies to the smallest of biotech companies, from government agencies to academic institutions – to commit to work together in addressing this dire crisis,” says Alfred Bourla, CEO.
Upon releasing the company’s 2019 financial results at the end of January 2020, Dr. Bourla stated, “2020 is expected to be an exciting year for Pfizer with the close of the Upjohn-Mylan transaction anticipated by mid-year, leaving New Pfizer positioned to deliver revenue and adjusted diluted EPS growth that is expected to be among the industry leaders. New Pfizer will be a smaller, science-based company with a singular focus on innovation while also continuing to allocate significant capital directly to shareholders, primarily through dividends.”
But then came COVID-19, delaying the formation of Viatris, and suddenly Pfizer found itself square in the battle against coronavirus as the lockdown began in March.
“In this troubling time, Pfizer is committed to doing all we can to respond to the COVID-19 pandemic,” Dr. Bourla said on March 13 when the company outlined a five-point plan to fight COVID-19. “Many companies, including Pfizer, are working to develop antiviral therapies to help infected patients fight this emerging virus as well as new vaccines to prevent infection and halt the further spread of this disease. Pfizer is working to advance our own potential antiviral therapies and is engaged with BioNTech on a potential mRNA coronavirus vaccine. We are committed to work as one team across the industry to harness our scientific expertise, technical skills and manufacturing capabilities to combat this evolving crisis.”
Pfizer promised to share tools and assets; marshal its people; apply the company’s drug-development expertise; offer its manufacturing capability; and improve future rapid response.
“In recent years, the biopharmaceutical industry has brought forward some of the most impactful medical breakthroughs known to society, from therapies for HIV and cancer that have extended millions of lives to novel gene therapies that are seeing cure-like outcomes for some the most devastating rare diseases,” Dr. Bourla says. “Pfizer calls on all members of the innovation ecosystem – from large pharmaceutical companies to the smallest of biotech companies, from government agencies to academic institutions – to commit to work together in addressing this dire crisis. With our combined efforts we know that there is no health challenge that we cannot overcome.”
In September, Dr. Bourla joined the CEOs of eight other pharmaceutical companies developing COVID-19 vaccines to continue to make the safety and well-being of vaccinated individuals the top priority. The other CEOs signing the pledge are from AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, and Sanofi.
The companies pledged to always make the safety and well-being of vaccinated individuals their top priority; continue to adhere to high scientific and ethical standards regarding the conduct of clinical trials and the rigor of manufacturing processes; only submit for approval or emergency use authorization after demonstrating safety and efficacy through a Phase III clinical study that is designed and conducted to meet requirements of expert regulatory authorities such as FDA; and work to ensure a sufficient supply and range of vaccine options, including those suitable for global access.
“We believe this pledge will help ensure public confidence in the rigorous scientific and regulatory process by which COVID-19 vaccines are evaluated and may ultimately be approved,” the CEOs stated.
The German phase of the COVID-19 vaccine trial with BioNTech began in April. By May, the U.S. phase of the trial started, with the first patients being dosed.
By July, the two companies had announced early positive data from a Phase I/II trial for BNT162b1, an MRNA-based vaccine candidate. At day 28 (seven days after dose two), all subjects who received 10 or 30 µg of BNT162b1 had significantly elevated RBD-binding IgG antibodies with geometric mean concentrations (GMCs) of 4,813 and 27,872 units/ml, which are eight and 46.3-times, respectively, the GMC of 602 units/ml in a panel of 38 sera of convalescent patients who had contracted SARS-CoV-2
At day 28 (seven days after dose two), all subjects who received 10 or 30 µg of BNT162b1 had SARS-CoV-2 neutralizing antibodies with geometric mean titers (GMTs) of 168 and 267, which are 1.8- and 2.8-times, respectively, the GMT of the convalescent serum panel.
Local reactions and systemic events after immunization with 10 µg and 30 µg of BNT162b1 were dose-dependent, generally mild to moderate, and transient. No serious adverse events were reported.
FDA granted Fast Track Designation to BNT162b1 and the candidate was advanced into global clinical studies in July. Pfizer and BioNTech also pledged to provide 20 million doses of the vaccine to the United Kingdom, 120 million to Japan, 200 million to the EU, and 600 million to the United States.
In September, Pfizer and BioNTech proposed an expansion of their Phase III pivotal COVID-19 vaccine trial to up to 44,000 participants, which allows for the enrollment of new populations. The proposed expansion would allow the companies to further increase trial population diversity, and include adolescents as young as 16 years of age and people with chronic, stable HIV (human immunodeficiency viruses), hepatitis C or hepatitis B infection, as well as provide additional safety and efficacy data.
Based on infection rates, at the time, the companies expected that a conclusive readout on efficacy was likely by the end of October 2020.
During August, Pfizer announced a multi-year agreement with Gilead Sciences Inc. to manufacture and supply Gilead’s investigational antiviral remdesivir, as one of multiple external manufacturing organizations supporting efforts to scale up supply of the investigational treatment for COVID-19. Under the terms of the agreement, Pfizer is providing contract manufacturing services at Pfizer’s McPherson, Kansas facility to manufacture and supply remdesivir for Gilead.
“From the beginning it was clear that no one company or innovation would be able to bring an end to the COVID-19 crisis. Pfizer’s agreement with Gilead is an excellent example of members of the innovation ecosystem working together to deliver medical solutions,” Dr. Bourla said on Aug. 7. “Together, we are more powerful than alone. As one of the largest manufacturers of vaccines, biologics and sterile injectables, it is a privilege to offer our expertise and infrastructure to help fight this pandemic. In that spirit, we are pleased that Gilead is using our manufacturing capacity to help facilitate supply of this medicine to patients as quickly as possible.”
Financial & Product Performances
Pfizer generated revenue of $51.75 billion in 2019, 4 percent less than in 2018. The decreased in revenue was attributed to the impact of the closing of the consumer health joint venture with GlaxoSmithKline, GSK Consumer Healthcare, in the third quarter of 2019. Pfizer owns a 32 percent equity stake in the joint venture and GSK owns 68 percent.
Pfizer’s 2019 net income was $16.27 billion, 46 percent more than in 2018. Diluted earnings per share were $2.87 compared with $1.87 in the prior year.
For the first half of 2020, revenue was $23.83 billion, 10 percent less than in the previous year’s period. Net income was $6.83 billion compared with $8.93 billion in first-half 2019. Diluted earnings were share for the 2020 first half were $1.22, 22 percent less than in first-half 2019. Pfizer attributes the declines to the impact of COVID-19 on the company’s sales and marketing activities due to widespread restrictions on in-person meetings with healthcare professionals and the refocused attention of the medical community on fighting the pandemic.
“Access to prescribers for sales force colleagues during second-quarter 2020 was mixed, with those in most international markets able to meet with healthcare professionals for most of the quarter, while those in the U.S. were unable to meet in-person with doctors for nearly all of the quarter,” Pfizer executives stated. “As a result of the lower number of in-person meetings with prescribers and restrictions on patient movements due to government-mandated work-from-home or shelter-in-place policies, the rate of new prescriptions for certain products and of vaccination rates for most vaccines slowed in certain markets, including the U.S., which negatively impacted second-quarter 2020 financial results. These declines were partially offset by certain Pfizer medicines and vaccines that saw increased demand in certain markets compared to the prior-year quarter, including Prevenar 13 for streptococcus pneumoniae in adults in international markets as well as certain sterile injectable products utilized in the intubation and ongoing treatment of mechanically ventilated COVID-19 patients.”
Pfizer’s top-selling franchise in 2019 for the fifth consecutive year was the Prevnar 13 vaccine family, which generated $5.85 billion, 1 percent more than in 2018. In first-half 2020, Prevnar 13/Prevenar 13 sales totaled $2.57 billion, down 4 percent versus same-period 2019.
The oncology drug Ibrance hopped into Pfizer’s No. 2 sales spot in 2019, generating $4.96 billion, 20 percent more than in 2018. In the first six months of 2020, Ibrance sales were $2.6 billion, 9 percent more than in first-half 2018.
Next in the 2019 sales line was the blood thinner Eliquis, for which Pfizer recorded alliance revenue and direct sales of $4.22 billion, representing 23 percent growth from 2018. During first-half 2020 the drug generated $2.57 billion in alliance revenue and direct sales for Pfizer, 23 percent more than in the first half of 2019.
No. 4 was Lyrica, which posted sales of $3.32 billion, 33 percent less than in 2019. Sales in the first half of 2020 totaled $706 million compared with $2.36 billion in the same period of the previous year.
Pfizer’s fifth best seller was the rheumatoid arthritis, psoriatic arthritis and ulcerative colitis drug Xeljanz, which made $2.24 billion in 2019, 26 percent more than in 2018. First-half 2020 sales were $1.09 billion, up 5 percent versus first-half 2019.
Once again at No. 6 in sales was the cholesterol drug Lipitor, generating $1.97 billion, 4 percent less than in 2018. Sales for first-half 2020 were $836 million, 19 percent less than in the first six months of 2019.
The rheumatoid arthritis drug Enbrel slipped to No. 7 in Pfizer sales for 2019, at $1.7 billion, 20 percent less than in 2018. In the first half of 2020, the company’s sales amounted to $684 million, a decline of 21 percent compared with first-half 2019.
Coming in at No. 8 was the smoking cessation drug Chantix/Champix, with $1.11 billion, an increase of 2 percent from 2018. Sales in the first half of 2020 were $505 million, 8 percent less than in first-half 2019.
At No. 9 was the hypertension drug Norvasc, with sales of $950 million, 8 percent less than in 2018. In the first half of 2020, sales were $419 million, 19 percent less than in the same period of the prior year.
The oncology drug Sutent placed No. 10 in sales in 2019, at $936 million, 11 percent less than in 2018. During the first half of 2020, Sutent sales declined 14 percent from first-half 2019 to $414 million.
Pfizer reported alliance revenue of $838 million for the oncology drug Xtandi in 2019, growing from $699 in 2018. First-half 2020 alliance revenue amounted to $475 million, 29 percent more than in first-half 2018.
The Premarin family of hormone replacement products was the 12th best seller in 2019, at $734 million, a decline of 12 percent. Sales in the first six months of 2020 were $304 million, 16 percent less than in the same period of 2019.
The anti-inflammatory drug Celebrex moved to N0. 13 on Pfizer’s 2019 best sellers list, with sales of $719 million, 5 percent more from the previous year. First-half 2020 sales were $295 million, 15 percent less than in first-half 2019.
No. 14 on Pfizer’s 2019 sales ladder was the combination antibiotic Sulperazon, which is used in the hospital setting. The product generated $684 million, 12 percent more than in 2018. First-half 2020 sales were $289 million, 16 percent less than in the same period of 2019.
N0. 15 in 2019 sales was the immunosuppressive drug Inflectra/Remsima at $625 million, 3 percent less than in 2018. Sales in the first six months of 2020 were $308 million, up 6 percent from first-half 2019.
No. 16 was the oncology drug Xalkori at $530 million, up 1 percent versus the 2018 amount. Sales in the first half of 2020 reached $287 million, 12 percent more than in first-half 2019.
R&D and the pipeline
Pfizer had a lot of R&D plans at the beginning of 2020. When presenting the company’s 2019 results, Dr. Bourla stated that in the first half of 2020, the company expected to report pivotal top-line results for the JADE Compare study for abrocitinib (PF-04965842), Pfizer’s Janus kinase-1 (JAK1) inhibitor for moderate-to-severe atopic dermatitis (AD); for three Phase III trials of PF-06482077, a 20-valent pneumococcal conjugate vaccine candidate in adults aged 18 and older; and for Xeljanz in ankylosing spondylitis, in addition to the potentially registration-enabling Phase II ANCHOR study evaluating the combination of Braftovi, Mektovi and cetuximab for the first-line treatment of BRAFV600E-mutant metastatic colorectal cancer.
The company also expected data in the first half of 2020 for promising earlier-stage opportunities, including proof-of-concept readouts for PF-06939926, a mini-dystrophin gene therapy candidate for Duchenne muscular dystrophy; for PF-06928316, a prophylactic vaccine candidate for the prevention of respiratory syncytial virus infection; and for PF-06700841, an investigational topical TYK2/ JAK1 dual inhibitor for psoriasis and AD.
In the second half of 2020, the company expected top-line results for the Phase III PENELOPE-B study of Ibrance in early-stage breast cancer, as well as for proof-of-concept readouts for PF-06651600, a dual JAK3/TEC inhibitor as a potential treatment for vitiligo; for PF-06700841 for the potential treatment of psoriatic arthritis (PsA); and for PF-06826647, an investigational TYK2 inhibitor for psoriasis.
COVID-19, however, threw some of these plans awry with what company executives described as a brief pause in the recruitment portion of certain ongoing clinical studies and a delay to most new study starts, However, in late April 2020, Pfizer restarted recruitment across the development portfolio, including new study starts.
“Pfizer continues to work closely with clinical trial sites to understand their needs and is performing remote monitoring where appropriate to oversee study conduct,” management says. “In addition, processes to enable tele-health and home healthcare are being utilized where appropriate to continue the data collection process and support patient safety.”
Pfizer kicked off 2020 with an announcement with development partner EMD Serono, the pharmaceuticals business of Merck KGaA, that the Phase III JAVELIN Bladder 100 study met its primary endpoint of overall survival (OS) at the planned interim analysis. In this study, patients with previously untreated locally advanced or metastatic urothelial carcinoma (UC) whose disease did not progress on induction chemotherapy and who were randomized to receive first-line maintenance therapy with Bavencio (avelumab) and best supportive care (BSC) lived significantly longer than those who received BSC only.
“Bavencio is the first immunotherapy to demonstrate in a clinical trial a statistically significant improvement in overall survival as a first-line treatment for patients with advanced urothelial carcinoma,” says Chris Boshoff, M.D., Ph.D., chief development officer, Oncology, Pfizer Global Product Development. “These latest positive data from the JAVELIN clinical development program add to the body of evidence for Bavencio in the treatment of genitourinary cancers, and we look forward to discussing these results with health authorities.”
In February, Astellas Pharma Inc. and Pfizer announced results of the final overall survival ß(OS) analysis from the Phase III PROSPER trial, which evaluated Xtandi (enzalutamide) plus androgen deprivation therapy (ADT) versus placebo plus ADT in men with non-metastatic castration-resistant prostate cancer (nmCRPC).
The results demonstrated a statistically significant improvement in OS in patients with nmCRPC who were treated with Xtandi plus ADT. OS was a key secondary endpoint of the trial. In a preliminary analysis, adverse events were generally consistent with those previously reported from PROSPER.
In May, the companies shared final results from this trial, which showed that Xtandi plus ADT reduced the risk of death by 27 percent (n=1,401; hazard ratio [HR]=0.73; [95% confidence interval [CI]: 0.61-0.89]; p=0.001) compared to placebo plus ADT. The median OS was 67.0 months (95 percent CI: 64.0 to not reached) for men who received Xtandi plus ADT compared to 56.3 months (95 percent CI: 54.4 to 63.0) with placebo plus ADT.
These data were simultaneously published online in the New England Journal of Medicine and presented during the virtual scientific program of the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting.
In March, FDA accepted for review a Biologics License Application (BLA) for tanezumab 2.5 mg administered subcutaneously (SC), which is being evaluated for patients with chronic pain due to moderate-to-severe osteoarthritis who have experienced inadequate pain relief with other analgesics. Tanezumab is a monoclonal antibody that is part of an investigational class of non-opioid chronic pain medications known as nerve growth factor (NGF) inhibitors.
The Prescription Drug User Fee Act (PDUFA) goal date for the FDA to make a decision on the tanezumab application is in December 2020.
Though good news came in January for one of the Bavencio trials, in March EMD Serono and Pfizer Inc. announced that they had accepted the recommendation of the independent data monitoring committee to terminate the Phase III JAVELIN Head and Neck 100 study. The trial was evaluating avelumab in addition to chemoradiotherapy (CRT) versus standard-of-care CRT in patients with untreated locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). The recommendation to terminate was made because the study is unlikely to show a statistically significant improvement in the primary endpoint of progression-free survival (PFS) based on a preplanned interim analysis.
In April, EMD Serono and Pfizer announced completion of the submission of a supplemental Biologics License Application (sBLA) to FDA for Bavencio for first-line maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma. The FDA granted Breakthrough Therapy Designation to Bavencio for this indication, and the sBLA was being reviewed by the FDA under its Real-Time Oncology Review (RTOR) pilot program.
The application is based on the positive results from an interim analysis of the Phase III JAVELIN Bladder 100 trial, preliminary results of which were announced during January. The interim analysis found that the trial met the primary endpoint of OS. Bavencio plus best supportive care as first-line maintenance therapy significantly extended the survival of patients with previously untreated locally advanced or metastatic UC whose disease did not progress on induction chemotherapy, compared with BSC only. A statistically significant improvement was demonstrated in both co-primary populations: all randomized patients and patients with PD-L1–positive tumors.
According to Dr. Boshoff, “Participation in the Real-Time Oncology Review program, coupled with the Breakthrough Therapy Designation, reflect the potential impact of Bavencio in this patient setting and provide the opportunity to work towards bringing this treatment option to patients as quickly as possible.”
The RTOR program is intended to create a more efficient review process to bring safe and effective treatments to patients earlier, including drugs that are likely to demonstrate substantial improvements over currently available therapy. The program allows the FDA to review clinical trial data from certain applications before the complete application is formally submitted.
FDA approved in June the sBLA for Bavencio in this indication. The agency previously approved Bavencio under the accelerated approval program in 2017 for the treatment of patients with locally advanced or metastatic UC who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy, based on tumor response rate and duration of response. Continued approval was contingent upon verification of clinical benefit, which was demonstrated in JAVELIN Bladder 100. The FDA converted the accelerated approval to full approval.
Pfizer announced in May that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion for the Hedgehog pathway inhibitor Daurismo. The CHMP recommended marketing authorization for Daurismo (glasdegib), in combination with low-dose cytarabine, a type of chemotherapy, for the treatment of newly diagnosed (denovo or secondary) acute myeloid leukemia in adult patients who are not candidates for standard chemotherapy. The CHMP’s opinion is being reviewed by the European Commission.
Also in May, Pfizer announced updated Phase 1b clinical data on PF-06939926, an investigational gene therapy being developed to treat Duchenne muscular dystrophy (DMD). The preliminary data from 9 ambulatory boys with DMD, aged 6 to 12 (mean age: 8 years) indicate that the intravenous administration of PF-06939926 was well-tolerated during the infusion period, with encouraging efficacy and manageable safety events, even when considering those adverse events that were more severe in nature. The treatment provided durable and statistically significant improvements across multiple efficacy-related endpoints measured at 12 months post-infusion, including sustained levels of mini-dystrophin expression and improvements on the North Star Ambulatory Assessment (NSAA) rating scale, which is a validated measure of muscle function. Three serious adverse events (SAEs) were recorded, two of which reflected likely complement activation. While these two SAEs were severe in nature, all three events fully resolved within two weeks, providing encouragement that close monitoring and early intervention can help mitigate the effects of complement activation. This new dataset, which includes updated 12-month results on safety, dystrophin expression, and exploratory functional endpoints for three additional boys, was presented for the first time at the American Society of Gene & Cell Therapy Annual Meeting.
“Based on the encouraging preliminary efficacy data and manageable safety events from our Phase 1b study, we believe we may have a potential breakthrough therapy for boys with Duchenne muscular dystrophy, a devastating disease for which there remains a significant medical need,” says Seng Cheng, Ph.D., chief scientific officer, Pfizer Rare Disease Research Unit. “We are advancing our Phase III program as quickly as possible and plan to begin dosing patients in the second half of 2020 pending regulatory approval. Our program has the potential to be the first DMD gene therapy Phase III trial start using a commercial-scale manufacturing process. If the program is successful, this manufacturing capability is expected to help position us to deliver this medicine to patients quickly following regulatory approval.”
Pfizer announced on Oct. 1, 2020, that PF-06939926 received Fast Track designation from the FDA for DMD.
Although Pfizer was expected to complete the PALLAS trial in early-stage breast cancer during early 2021, the month of May instead brought bad news. The independent data monitoring committee determined that the clinical trial is unlikely to show a statistically significant improvement in the primary endpoint of invasive disease-free survival (iDFS). The PALLAS trial compared palbociclib plus standard adjuvant endocrine therapy to standard adjuvant endocrine therapy alone in women and men with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) early (stage 2 and 3) breast cancer.
In September, FDA accepted and granted priority review to the supplemental New Drug Application (sNDA) for Xalkori (crizotinib) for the treatment of pediatric patients with relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) that is anaplastic lymphoma kinase (ALK)-positive. Xalkori received Breakthrough Therapy Designation (BTD) for the ALK-positive ALCL indication in May 2018 and if approved, would be the first biomarker-driven therapy for this type of pediatric lymphoma. The PDUFA goal date for a decision by the FDA is January 2021.
“Despite high survival rates for children with ALK-positive anaplastic large cell lymphoma, many will relapse, requiring novel treatment approaches,” Dr. Boshoff says, adding that the sNDA filing exemplifies Pfizer’s commitment to broadening the use of biomarker-driven therapies in areas with significant needs, such as rare, pediatric cancers.
“Given Xalkori’s proven efficacy in ALK-positive lung cancer and activity seen in clinical trials investigating relapsed or refractory ALK- and ROS-1 positive anaplastic large cell lymphoma, if approved, Xalkori could represent an important step toward improving outcomes for children with this type of cancer,” Boschoff says.
In August, Pfizer announced that the CROWN study of Lorbrena (lorlatinib) in people with previously untreated advanced ALK-positive NSCLC met its primary endpoint by demonstrating significantly improved PFS, as compared to Xalkori. The results were reviewed by an independent data monitoring committee at a planned interim analysis. The safety profile for lorlatinib and crizotinib were consistent with what has been previously seen in clinical trials.
“Almost a decade ago, we pioneered the first biomarker-driven medicine for ALK-positive non-small cell lung cancer, which transformed treatment of this disease,” Dr. Boshoff says. “These top-line results of the CROWN study reinforce the significant benefit of Lorbrena demonstrated in later-line settings, and we are excited to share these data soon with physicians and other healthcare providers, as well as engage with global regulatory authorities to potentially provide people with previously untreated metastatic non-small cell lung cancer this third-generation ALK inhibitor.”
In 2018, FDA approved Lorbrena for the treatment of patients with ALK-positive metastatic NSCLC whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease; or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease.
The FDA on Sept. 25, 2020, approved Xeljanz for treating children and adolescents 2 years and older with active polyarticular course juvenile idiopathic arthritis (pcJIA). Two formulations were approved, a tablet and an oral solution, which are dosed based upon weight. Xeljanz is the first FDA-approved JAK inhibitor for treating pcJIA.
International
Four Years Ago This Week, Freedom Was Torched
Four Years Ago This Week, Freedom Was Torched
Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare…

Authored by Jeffrey Tucker via The Brownstone Institute,
"Beware the Ides of March,” Shakespeare quotes the soothsayer’s warning Julius Caesar about what turned out to be an impending assassination on March 15. The death of American liberty happened around the same time four years ago, when the orders went out from all levels of government to close all indoor and outdoor venues where people gather.
It was not quite a law and it was never voted on by anyone. Seemingly out of nowhere, people who the public had largely ignored, the public health bureaucrats, all united to tell the executives in charge – mayors, governors, and the president – that the only way to deal with a respiratory virus was to scrap freedom and the Bill of Rights.
And they did, not only in the US but all over the world.
The forced closures in the US began on March 6 when the mayor of Austin, Texas, announced the shutdown of the technology and arts festival South by Southwest. Hundreds of thousands of contracts, of attendees and vendors, were instantly scrapped. The mayor said he was acting on the advice of his health experts and they in turn pointed to the CDC, which in turn pointed to the World Health Organization, which in turn pointed to member states and so on.
There was no record of Covid in Austin, Texas, that day but they were sure they were doing their part to stop the spread. It was the first deployment of the “Zero Covid” strategy that became, for a time, official US policy, just as in China.
It was never clear precisely who to blame or who would take responsibility, legal or otherwise.
This Friday evening press conference in Austin was just the beginning. By the next Thursday evening, the lockdown mania reached a full crescendo. Donald Trump went on nationwide television to announce that everything was under control but that he was stopping all travel in and out of US borders, from Europe, the UK, Australia, and New Zealand. American citizens would need to return by Monday or be stuck.
Americans abroad panicked while spending on tickets home and crowded into international airports with waits up to 8 hours standing shoulder to shoulder. It was the first clear sign: there would be no consistency in the deployment of these edicts.
There is no historical record of any American president ever issuing global travel restrictions like this without a declaration of war. Until then, and since the age of travel began, every American had taken it for granted that he could buy a ticket and board a plane. That was no longer possible. Very quickly it became even difficult to travel state to state, as most states eventually implemented a two-week quarantine rule.
The next day, Friday March 13, Broadway closed and New York City began to empty out as any residents who could went to summer homes or out of state.
On that day, the Trump administration declared the national emergency by invoking the Stafford Act which triggers new powers and resources to the Federal Emergency Management Administration.
In addition, the Department of Health and Human Services issued a classified document, only to be released to the public months later. The document initiated the lockdowns. It still does not exist on any government website.
The White House Coronavirus Response Task Force, led by the Vice President, will coordinate a whole-of-government approach, including governors, state and local officials, and members of Congress, to develop the best options for the safety, well-being, and health of the American people. HHS is the LFA [Lead Federal Agency] for coordinating the federal response to COVID-19.
Closures were guaranteed:
Recommend significantly limiting public gatherings and cancellation of almost all sporting events, performances, and public and private meetings that cannot be convened by phone. Consider school closures. Issue widespread ‘stay at home’ directives for public and private organizations, with nearly 100% telework for some, although critical public services and infrastructure may need to retain skeleton crews. Law enforcement could shift to focus more on crime prevention, as routine monitoring of storefronts could be important.
In this vision of turnkey totalitarian control of society, the vaccine was pre-approved: “Partner with pharmaceutical industry to produce anti-virals and vaccine.”
The National Security Council was put in charge of policy making. The CDC was just the marketing operation. That’s why it felt like martial law. Without using those words, that’s what was being declared. It even urged information management, with censorship strongly implied.
The timing here is fascinating. This document came out on a Friday. But according to every autobiographical account – from Mike Pence and Scott Gottlieb to Deborah Birx and Jared Kushner – the gathered team did not meet with Trump himself until the weekend of the 14th and 15th, Saturday and Sunday.
According to their account, this was his first real encounter with the urge that he lock down the whole country. He reluctantly agreed to 15 days to flatten the curve. He announced this on Monday the 16th with the famous line: “All public and private venues where people gather should be closed.”
This makes no sense. The decision had already been made and all enabling documents were already in circulation.
There are only two possibilities.
One: the Department of Homeland Security issued this March 13 HHS document without Trump’s knowledge or authority. That seems unlikely.
Two: Kushner, Birx, Pence, and Gottlieb are lying. They decided on a story and they are sticking to it.
Trump himself has never explained the timeline or precisely when he decided to greenlight the lockdowns. To this day, he avoids the issue beyond his constant claim that he doesn’t get enough credit for his handling of the pandemic.
With Nixon, the famous question was always what did he know and when did he know it? When it comes to Trump and insofar as concerns Covid lockdowns – unlike the fake allegations of collusion with Russia – we have no investigations. To this day, no one in the corporate media seems even slightly interested in why, how, or when human rights got abolished by bureaucratic edict.
As part of the lockdowns, the Cybersecurity and Infrastructure Security Agency, which was and is part of the Department of Homeland Security, as set up in 2018, broke the entire American labor force into essential and nonessential.
They also set up and enforced censorship protocols, which is why it seemed like so few objected. In addition, CISA was tasked with overseeing mail-in ballots.
Only 8 days into the 15, Trump announced that he wanted to open the country by Easter, which was on April 12. His announcement on March 24 was treated as outrageous and irresponsible by the national press but keep in mind: Easter would already take us beyond the initial two-week lockdown. What seemed to be an opening was an extension of closing.
This announcement by Trump encouraged Birx and Fauci to ask for an additional 30 days of lockdown, which Trump granted. Even on April 23, Trump told Georgia and Florida, which had made noises about reopening, that “It’s too soon.” He publicly fought with the governor of Georgia, who was first to open his state.
Before the 15 days was over, Congress passed and the president signed the 880-page CARES Act, which authorized the distribution of $2 trillion to states, businesses, and individuals, thus guaranteeing that lockdowns would continue for the duration.
There was never a stated exit plan beyond Birx’s public statements that she wanted zero cases of Covid in the country. That was never going to happen. It is very likely that the virus had already been circulating in the US and Canada from October 2019. A famous seroprevalence study by Jay Bhattacharya came out in May 2020 discerning that infections and immunity were already widespread in the California county they examined.
What that implied was two crucial points: there was zero hope for the Zero Covid mission and this pandemic would end as they all did, through endemicity via exposure, not from a vaccine as such. That was certainly not the message that was being broadcast from Washington. The growing sense at the time was that we all had to sit tight and just wait for the inoculation on which pharmaceutical companies were working.
By summer 2020, you recall what happened. A restless generation of kids fed up with this stay-at-home nonsense seized on the opportunity to protest racial injustice in the killing of George Floyd. Public health officials approved of these gatherings – unlike protests against lockdowns – on grounds that racism was a virus even more serious than Covid. Some of these protests got out of hand and became violent and destructive.
Meanwhile, substance abuse rage – the liquor and weed stores never closed – and immune systems were being degraded by lack of normal exposure, exactly as the Bakersfield doctors had predicted. Millions of small businesses had closed. The learning losses from school closures were mounting, as it turned out that Zoom school was near worthless.
It was about this time that Trump seemed to figure out – thanks to the wise council of Dr. Scott Atlas – that he had been played and started urging states to reopen. But it was strange: he seemed to be less in the position of being a president in charge and more of a public pundit, Tweeting out his wishes until his account was banned. He was unable to put the worms back in the can that he had approved opening.
By that time, and by all accounts, Trump was convinced that the whole effort was a mistake, that he had been trolled into wrecking the country he promised to make great. It was too late. Mail-in ballots had been widely approved, the country was in shambles, the media and public health bureaucrats were ruling the airwaves, and his final months of the campaign failed even to come to grips with the reality on the ground.
At the time, many people had predicted that once Biden took office and the vaccine was released, Covid would be declared to have been beaten. But that didn’t happen and mainly for one reason: resistance to the vaccine was more intense than anyone had predicted. The Biden administration attempted to impose mandates on the entire US workforce. Thanks to a Supreme Court ruling, that effort was thwarted but not before HR departments around the country had already implemented them.
As the months rolled on – and four major cities closed all public accommodations to the unvaccinated, who were being demonized for prolonging the pandemic – it became clear that the vaccine could not and would not stop infection or transmission, which means that this shot could not be classified as a public health benefit. Even as a private benefit, the evidence was mixed. Any protection it provided was short-lived and reports of vaccine injury began to mount. Even now, we cannot gain full clarity on the scale of the problem because essential data and documentation remains classified.
After four years, we find ourselves in a strange position. We still do not know precisely what unfolded in mid-March 2020: who made what decisions, when, and why. There has been no serious attempt at any high level to provide a clear accounting much less assign blame.
Not even Tucker Carlson, who reportedly played a crucial role in getting Trump to panic over the virus, will tell us the source of his own information or what his source told him. There have been a series of valuable hearings in the House and Senate but they have received little to no press attention, and none have focus on the lockdown orders themselves.
The prevailing attitude in public life is just to forget the whole thing. And yet we live now in a country very different from the one we inhabited five years ago. Our media is captured. Social media is widely censored in violation of the First Amendment, a problem being taken up by the Supreme Court this month with no certainty of the outcome. The administrative state that seized control has not given up power. Crime has been normalized. Art and music institutions are on the rocks. Public trust in all official institutions is at rock bottom. We don’t even know if we can trust the elections anymore.
In the early days of lockdown, Henry Kissinger warned that if the mitigation plan does not go well, the world will find itself set “on fire.” He died in 2023. Meanwhile, the world is indeed on fire. The essential struggle in every country on earth today concerns the battle between the authority and power of permanent administration apparatus of the state – the very one that took total control in lockdowns – and the enlightenment ideal of a government that is responsible to the will of the people and the moral demand for freedom and rights.
How this struggle turns out is the essential story of our times.
CODA: I’m embedding a copy of PanCAP Adapted, as annotated by Debbie Lerman. You might need to download the whole thing to see the annotations. If you can help with research, please do.
* * *
Jeffrey Tucker is the author of the excellent new book 'Life After Lock-Down'
Government
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
Authored by Jack Phillips via The Epoch Times (emphasis ours),
A…

Authored by Jack Phillips via The Epoch Times (emphasis ours),
A U.S. Centers for Disease Control (CDC) paper released Thursday found that thousands of young children have been taken to the emergency room over the past several years after taking the very common sleep-aid supplement melatonin.
The agency said that melatonin, which can come in gummies that are meant for adults, was implicated in about 7 percent of all emergency room visits for young children and infants “for unsupervised medication ingestions,” adding that many incidents were linked to the ingestion of gummy formulations that were flavored. Those incidents occurred between the years 2019 and 2022.
Melatonin is a hormone produced by the human body to regulate its sleep cycle. Supplements, which are sold in a number of different formulas, are generally taken before falling asleep and are popular among people suffering from insomnia, jet lag, chronic pain, or other problems.
The supplement isn’t regulated by the U.S. Food and Drug Administration and does not require child-resistant packaging. However, a number of supplement companies include caps or lids that are difficult for children to open.
The CDC report said that a significant number of melatonin-ingestion cases among young children were due to the children opening bottles that had not been properly closed or were within their reach. Thursday’s report, the agency said, “highlights the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight,” including melatonin.
The approximately 11,000 emergency department visits for unsupervised melatonin ingestions by infants and young children during 2019–2022 highlight the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight.
The CDC notes that melatonin use among Americans has increased five-fold over the past 25 years or so. That has coincided with a 530 percent increase in poison center calls for melatonin exposures to children between 2012 and 2021, it said, as well as a 420 percent increase in emergency visits for unsupervised melatonin ingestion by young children or infants between 2009 and 2020.
Some health officials advise that children under the age of 3 should avoid taking melatonin unless a doctor says otherwise. Side effects include drowsiness, headaches, agitation, dizziness, and bed wetting.
Other symptoms of too much melatonin include nausea, diarrhea, joint pain, anxiety, and irritability. The supplement can also impact blood pressure.
However, there is no established threshold for a melatonin overdose, officials have said. Most adult melatonin supplements contain a maximum of 10 milligrams of melatonin per serving, and some contain less.
Many people can tolerate even relatively large doses of melatonin without significant harm, officials say. But there is no antidote for an overdose. In cases of a child accidentally ingesting melatonin, doctors often ask a reliable adult to monitor them at home.
Dr. Cora Collette Breuner, with the Seattle Children’s Hospital at the University of Washington, told CNN that parents should speak with a doctor before giving their children the supplement.
“I also tell families, this is not something your child should take forever. Nobody knows what the long-term effects of taking this is on your child’s growth and development,” she told the outlet. “Taking away blue-light-emitting smartphones, tablets, laptops, and television at least two hours before bed will keep melatonin production humming along, as will reading or listening to bedtime stories in a softly lit room, taking a warm bath, or doing light stretches.”
In 2022, researchers found that in 2021, U.S. poison control centers received more than 52,000 calls about children consuming worrisome amounts of the dietary supplement. That’s a six-fold increase from about a decade earlier. Most such calls are about young children who accidentally got into bottles of melatonin, some of which come in the form of gummies for kids, the report said.
Dr. Karima Lelak, an emergency physician at Children’s Hospital of Michigan and the lead author of the study published in 2022 by the CDC, found that in about 83 percent of those calls, the children did not show any symptoms.
However, other children had vomiting, altered breathing, or other symptoms. Over the 10 years studied, more than 4,000 children were hospitalized, five were put on machines to help them breathe, and two children under the age of two died. Most of the hospitalized children were teenagers, and many of those ingestions were thought to be suicide attempts.
Those researchers also suggested that COVID-19 lockdowns and virtual learning forced more children to be at home all day, meaning there were more opportunities for kids to access melatonin. Also, those restrictions may have caused sleep-disrupting stress and anxiety, leading more families to consider melatonin, they suggested.
The Associated Press contributed to this report.
International
Red Candle In The Wind
Red Candle In The Wind
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by…
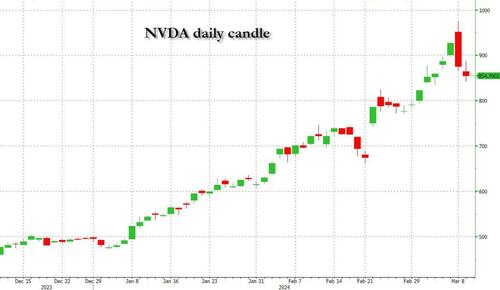
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by printing at 275,000 against a consensus call of 200,000. We say superficially, because the downward revisions to prior months totalled 167,000 for December and January, taking the total change in employed persons well below the implied forecast, and helping the unemployment rate to pop two-ticks to 3.9%. The U6 underemployment rate also rose from 7.2% to 7.3%, while average hourly earnings growth fell to 0.2% m-o-m and average weekly hours worked languished at 34.3, equalling pre-pandemic lows.
Undeterred by the devil in the detail, the algos sprang into action once exchanges opened. Market darling NVIDIA hit a new intraday high of $974 before (presumably) the humans took over and sold the stock down more than 10% to close at $875.28. If our suspicions are correct that it was the AIs buying before the humans started selling (no doubt triggering trailing stops on the way down), the irony is not lost on us.
The 1-day chart for NVIDIA now makes for interesting viewing, because the red candle posted on Friday presents quite a strong bearish engulfing signal. Volume traded on the day was almost double the 15-day simple moving average, and similar price action is observable on the 1-day charts for both Intel and AMD. Regular readers will be aware that we have expressed incredulity in the past about the durability the AI thematic melt-up, so it will be interesting to see whether Friday’s sell off is just a profit-taking blip, or a genuine trend reversal.
AI equities aside, this week ought to be important for markets because the BTFP program expires today. That means that the Fed will no longer be loaning cash to the banking system in exchange for collateral pledged at-par. The KBW Regional Banking index has so far taken this in its stride and is trading 30% above the lows established during the mini banking crisis of this time last year, but the Fed’s liquidity facility was effectively an exercise in can-kicking that makes regional banks a sector of the market worth paying attention to in the weeks ahead. Even here in Sydney, regulators are warning of external risks posed to the banking sector from scheduled refinancing of commercial real estate loans following sharp falls in valuations.
Markets are sending signals in other sectors, too. Gold closed at a new record-high of $2178/oz on Friday after trading above $2200/oz briefly. Gold has been going ballistic since the Friday before last, posting gains even on days where 2-year Treasury yields have risen. Gold bugs are buying as real yields fall from the October highs and inflation breakevens creep higher. This is particularly interesting as gold ETFs have been recording net outflows; suggesting that price gains aren’t being driven by a retail pile-in. Are gold buyers now betting on a stagflationary outcome where the Fed cuts without inflation being anchored at the 2% target? The price action around the US CPI release tomorrow ought to be illuminating.
Leaving the day-to-day movements to one side, we are also seeing further signs of structural change at the macro level. The UK budget last week included a provision for the creation of a British ISA. That is, an Individual Savings Account that provides tax breaks to savers who invest their money in the stock of British companies. This follows moves last year to encourage pension funds to head up the risk curve by allocating 5% of their capital to unlisted investments.
As a Hail Mary option for a government cruising toward an electoral drubbing it’s a curious choice, but it’s worth highlighting as cash-strapped governments increasingly see private savings pools as a funding solution for their spending priorities.
Of course, the UK is not alone in making creeping moves towards financial repression. In contrast to announcements today of increased trade liberalisation, Australian Treasurer Jim Chalmers has in the recent past flagged his interest in tapping private pension savings to fund state spending priorities, including defence, public housing and renewable energy projects. Both the UK and Australia appear intent on finding ways to open up the lungs of their economies, but government wants more say in directing private capital flows for state goals.
So, how far is the blurring of the lines between free markets and state planning likely to go? Given the immense and varied budgetary (and security) pressures that governments are facing, could we see a re-up of WWII-era Victory bonds, where private investors are encouraged to do their patriotic duty by directly financing government at negative real rates?
That would really light a fire under the gold market.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International4 days ago
International4 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges






















