Government
New Drugs May Help Rebuild Momentum against Cardiovascular Disease
Gene therapies, antibody fragments, and microRNA drug products are being developed to counter cardiovascular disease mechanisms
The post New Drugs May…
By Tiffany Yesavage, PhD
We can gauge progress against cardiovascular disease by poring over statistics. But which statistics? We hear that cardiovascular disease is still the leading cause of death, but by less of a margin than previously. The narrowing of cardiovascular disease’s relative lead sounds encouraging, especially since the trend has held for about 50 years. Still, we may wonder if cardiovascular disease is receding or if other causes of death, such as cancer and respiratory disease, are advancing.
Perhaps a more telling statistic is premature heart disease mortality. It was front and center in a recent study led by researchers based at the U.S. Centers for Disease Control and Prevention (Trends Cardiovasc Med. 2020; 30(6): 364–374). The researchers indicated that the premature heart disease mortality rate among adults aged 25–64 had decreased by 70% since 1968, but that it had remained “stagnant” since 2011. Worse, by the end of the study period, heart disease still accounted for almost one in five of all deaths in the selected age group.
Essentially, the researchers told a story of momentum gained, then lost. Nonetheless, the researchers sounded an optimistic note. They wrote, “Public health and clinical systems can implement proven effective strategies and innovative promising practices across the spectrum of heart disease prevention.”
In their article, the researchers emphasized the proven strategies. For example, they cited the identification and management of key cardiovascular disease risk factors, as well as advances in medical treatment. The latter include the widespread adoption of cholesterol-lowering statins, arterial stents, and thrombolytic agents to break up blood clots.
Less was said about new innovations. But these merit attention, too. So, let’s look at just a few of them. Continue reading this GEN article to learn what four pioneering companies are doing to help the medical establishment rebuild momentum against heart disease and cardiovascular disease in general.
Targeted delivery of gene therapy to heart cells
Sharif Tabebordbar, PhD, chief scientific officer of Kate Therapeutics, recalls watching his father battle a rare genetic muscle disease called facioscapulohumeral muscular dystrophy. He remembers that the struggle, which lasted years, prompted him to think, “How can we make life better for my dad?” The experience inspired him to study genetics.
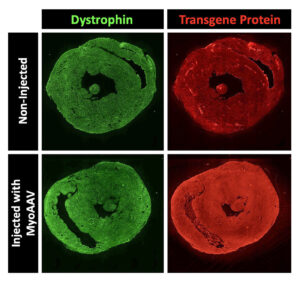
Nowadays, Tabebordbar hopes to improve the delivery of gene therapy. He focuses on altering the protein shell—called a capsid—of adeno-associated viruses (AAVs). These nonpathogenic viruses serve as vectors for transporting gene therapy to various tissues in the body. Each viral particle contains the cargo—the gene you want to deliver.
However, the main issue with injecting naturally occurring AAV capsids into the bloodstream is that most of these viruses are shuttled to the liver tissue. “Only a small percentage will actually get to the muscles or heart tissue,” Tabebordbar observes. “Therefore, you need very high levels of virus to have an effect on those tissues.” Unfortunately, elevated viral doses have been associated with severe liver toxicity and even death in recent clinical trials.
Over the past few years, there has been a significant focus on developing new modalities that will deliver gene therapy to specific tissues. In the case of naturally occurring AAVs, the proteins that form their surface structure determine which tissues of the body they can enter. These proteins can be modified and engineered using a technology called directed evolution.
Tabebordbar says that Kate Therapeutics is leveraging directed evolution approaches to engineer AAV capsids targeting muscles and heart tissues. However, he warns that an additional challenge with cardiovascular gene therapy involves ensuring homogenous delivery into heart muscle cells. “You don’t want drugs in just one part of the heart and not the other parts,” he stresses. “The pumping and electrical functions of the heart depend on the consistent, synchronized activity of all cardiomyocytes.”
Kate Therapeutics hasn’t disclosed the exact modalities and indications it is pursuing. Nonetheless, the company indicates that it aims to treat several rare genetic diseases. According to Tabebordbar, Kate Therapeutics will realize its goals by leveraging its ability to deliver gene therapy at a much lower dose. “This is going to result in much better safety profiles,” he declares. “That way, we can achieve the same gene expression levels in heart and muscle cells without having to worry about liver toxicity.”
Antithrombotic effects without bleeding
Platelets are tiny cell fragments in the blood responsible for natural coagulation, the process that stops cuts from bleeding. In contrast, cerebral thrombosis is the pathological mechanism of coagulation—in the form of a blood clot—that manifests during ischemic strokes.
“Unfortunately, most drugs that inhibit platelet aggregation also tend to cause bleeding,” says Gilles Avenard, MD, the CEO of Acticor Biotech. He explains that Acticor has developed a novel drug called glenzocimab—a humanized monoclonal antibody fragment—that disaggregates platelets without associated bleeding.

Glenzocimab is designed to inhibit a receptor on the surface of platelets called glycoprotein VI (GPVI), which binds to polymerized collagen and fibrin and promotes coagulation. In mice, deficiencies in GPVI have been found to be protective against thrombosis.
“The beauty of our drug,” Avenard maintains, “is that it targets thrombosis while also reducing inflammation downstream of the blood clot. This inflammation is thought to be due to small aggregates that dissolve the blood-brain barrier downstream of the clot, leading to intracerebral hemorrhages.”
Avenard explains that there are two major treatments for acute ischemic stroke: the administration of a drug called tissue plasminogen activator (tPA) and a procedure called a mechanical thrombectomy, which involves using a catheter to pull clots out of the cerebral artery. However, these treatments have both been associated with an increased risk of intracerebral hemorrhage.
Avenard reports that during Phase I/II trials to assess the safety of glenzocimab on top of tPA, investigators were pleasantly surprised to see that the treated group experienced fewer intracerebral hemorrhages than the placebo group. A Phase II/III study also involved administering the drug on top of tPA and thrombectomy. Whereas none of the patients in the treatment group died of an intracerebral hemorrhage, four patients in the placebo group (tPA and thrombectomy alone) died from bleeding.
According to Avenard, investigators expect to see similarly favorable results in a larger upcoming trial involving 1,000 patients worldwide. In addition to ischemic strokes, Acticor is planning future trials to target heart attacks and pulmonary embolisms.
Targeting microRNA to treat heart failure
Although noncoding RNA is not translated into proteins, it plays a crucial role in regulating many cellular processes. Notably, the dysregulation of noncoding RNA has been associated with human diseases, including cancer and heart failure.
Noncoding RNA figures in the work of Cardior Pharmaceuticals. “We have identified a crucial noncoding RNA called microRNA-132 that is responsible for the detrimental remodeling of heart cells,” says Thomas Thum, MD, PhD, the company’s co-founder and chief scientific officer. “We have documented how increases in microRNA-132 drive cardiac disease processes leading to heart failure.”

Thum explains that Cardior has developed CDR132L, a microRNA-132 inhibitor that specifically recognizes and “blocks” aberrant microRNA-132 levels. “CDR132L reverses cellular pathology, restoring normal heart muscle cells and overall heart function,” he explains. Thus far, the disease-modifying effects of the drug have been demonstrated in several animal models and a Phase Ib trial in chronic heart failure patients.
Cardior is also launching two Phase II trials to further assess the safety and efficacy of CDR132L. One trial is already enrolling participants with reduced left ventricular ejection fraction following a heart attack—an indication that the heart muscles are not pumping as well as normal. The other Phase II trial, set to begin enrollment in 2024, will investigate chronic heart failure in U.S. patients.
Thum stresses the importance of monitoring ongoing and future trials for the possibility of drug toxicity. Nevertheless, he says that the company’s Phase Ib study, conducted on top of the standard of care, showed no safety concerns or unexpected adverse effects, even at higher doses. He asserts, “This outcome, in addition to our extensive preclinical research into safety, supports our belief that CDR132L is a safe treatment option.”
Limiting tissue damage during heart attacks
During a heart attack, a blood clot forms within a coronary artery, reducing blood supply to specific areas of the heart. The affected region then becomes vulnerable to developing an infarct—a localized region of dead tissue caused by insufficient blood circulation.
Heart attacks prompt a series of reactions in the body referred to as adrenergic stress, leading to increases in heart rates and the force of the heartbeat—known as contractility. And while stenting procedures often help to widen constricted coronary arteries associated with heart attacks, they also increase adrenergic stress.
Kjetil Hestdal, MD, PhD, the CEO of Serca Pharmaceuticals, explains that the company is developing a drug called 13-M that disables the effects of adrenergic overstimulation in the heart: “By reducing contractility, the heart’s muscle cells in the vulnerable area surrounding the infarct will experience a lower resting workload. This will reduce the overall energy demand and prevent the death of these cells.” He also emphasizes that reductions in infarct size are correlated with a lower risk of developing heart failure following a heart attack.
Hestdal says that 13-M—currently in the preclinical development stage—is a new chemical entity that came from the laboratory of Kjetil Taskén, MD, PhD, a professor of medicine at the University of Oslo. Taskén’s team developed a sensitive, high-throughput assay to screen over 80,000 compounds for the ability to block a critical pathway in the adrenergic stimulation of heart muscle called SERCA2. “The SERCA2 pump,” Hestdal points out, “regulates calcium flow in the heart muscle cells and plays a key role in myocardial contractility.”
Hestdal says that 13-M will be administered intravenously before and after stenting procedures. He adds, “To our knowledge, no other drug in development has the same cardioprotective target.” He explains that the key behind 13-M is that it works by modulating contractility while maintaining heart rate: “This combination reduces the chances of inducing unintended disturbances in heart rate that could potentially lead to serious heart arrhythmias or heart fibrillation.” In contrast, drugs such as beta blockers lower both heart rate and contractility.
Looking toward the future
Avenard stresses that although emerging therapies like gene editing are very exciting, emergency treatments will always be necessary for heart attacks and strokes. Meanwhile, Thum argues that many cardiovascular drugs on the market today tend to alleviate symptoms without addressing underlying root causes. He notes, however, that “with new treatment modalities like microRNA therapy, we can target downstream pathways that regulate critical processes in the pathology.”
Finally, Tabebordbar says that Kate Therapeutics is hoping to target several genetic diseases over the next few years, including his father’s condition. Although he acknowledges that “there are still a lot of safety concerns to overcome,” he emphasizes that “gene therapy and gene editing ultimately have the potential to treat broader cardiovascular conditions like high cholesterol and heart failure.”
The post New Drugs May Help Rebuild Momentum against Cardiovascular Disease appeared first on GEN - Genetic Engineering and Biotechnology News.
disease control treatment clinical trials preclinical genetic therapy rnaGovernment
Are Voters Recoiling Against Disorder?
Are Voters Recoiling Against Disorder?
Authored by Michael Barone via The Epoch Times (emphasis ours),
The headlines coming out of the Super…

Authored by Michael Barone via The Epoch Times (emphasis ours),
The headlines coming out of the Super Tuesday primaries have got it right. Barring cataclysmic changes, Donald Trump and Joe Biden will be the Republican and Democratic nominees for president in 2024.
With Nikki Haley’s withdrawal, there will be no more significantly contested primaries or caucuses—the earliest both parties’ races have been over since something like the current primary-dominated system was put in place in 1972.
The primary results have spotlighted some of both nominees’ weaknesses.
Donald Trump lost high-income, high-educated constituencies, including the entire metro area—aka the Swamp. Many but by no means all Haley votes there were cast by Biden Democrats. Mr. Trump can’t afford to lose too many of the others in target states like Pennsylvania and Michigan.
Majorities and large minorities of voters in overwhelmingly Latino counties in Texas’s Rio Grande Valley and some in Houston voted against Joe Biden, and even more against Senate nominee Rep. Colin Allred (D-Texas).
Returns from Hispanic precincts in New Hampshire and Massachusetts show the same thing. Mr. Biden can’t afford to lose too many Latino votes in target states like Arizona and Georgia.
When Mr. Trump rode down that escalator in 2015, commentators assumed he’d repel Latinos. Instead, Latino voters nationally, and especially the closest eyewitnesses of Biden’s open-border policy, have been trending heavily Republican.
High-income liberal Democrats may sport lawn signs proclaiming, “In this house, we believe ... no human is illegal.” The logical consequence of that belief is an open border. But modest-income folks in border counties know that flows of illegal immigrants result in disorder, disease, and crime.
There is plenty of impatience with increased disorder in election returns below the presidential level. Consider Los Angeles County, America’s largest county, with nearly 10 million people, more people than 40 of the 50 states. It voted 71 percent for Mr. Biden in 2020.
Current returns show county District Attorney George Gascon winning only 21 percent of the vote in the nonpartisan primary. He’ll apparently face Republican Nathan Hochman, a critic of his liberal policies, in November.
Gascon, elected after the May 2020 death of counterfeit-passing suspect George Floyd in Minneapolis, is one of many county prosecutors supported by billionaire George Soros. His policies include not charging juveniles as adults, not seeking higher penalties for gang membership or use of firearms, and bringing fewer misdemeanor cases.
The predictable result has been increased car thefts, burglaries, and personal robberies. Some 120 assistant district attorneys have left the office, and there’s a backlog of 10,000 unprosecuted cases.
More than a dozen other Soros-backed and similarly liberal prosecutors have faced strong opposition or have left office.
St. Louis prosecutor Kim Gardner resigned last May amid lawsuits seeking her removal, Milwaukee’s John Chisholm retired in January, and Baltimore’s Marilyn Mosby was defeated in July 2022 and convicted of perjury in September 2023. Last November, Loudoun County, Virginia, voters (62 percent Biden) ousted liberal Buta Biberaj, who declined to prosecute a transgender student for assault, and in June 2022 voters in San Francisco (85 percent Biden) recalled famed radical Chesa Boudin.
Similarly, this Tuesday, voters in San Francisco passed ballot measures strengthening police powers and requiring treatment of drug-addicted welfare recipients.
In retrospect, it appears the Floyd video, appearing after three months of COVID-19 confinement, sparked a frenzied, even crazed reaction, especially among the highly educated and articulate. One fatal incident was seen as proof that America’s “systemic racism” was worse than ever and that police forces should be defunded and perhaps abolished.
2020 was “the year America went crazy,” I wrote in January 2021, a year in which police funding was actually cut by Democrats in New York, Los Angeles, San Francisco, Seattle, and Denver. A year in which young New York Times (NYT) staffers claimed they were endangered by the publication of Sen. Tom Cotton’s (R-Ark.) opinion article advocating calling in military forces if necessary to stop rioting, as had been done in Detroit in 1967 and Los Angeles in 1992. A craven NYT publisher even fired the editorial page editor for running the article.
Evidence of visible and tangible discontent with increasing violence and its consequences—barren and locked shelves in Manhattan chain drugstores, skyrocketing carjackings in Washington, D.C.—is as unmistakable in polls and election results as it is in daily life in large metropolitan areas. Maybe 2024 will turn out to be the year even liberal America stopped acting crazy.
Chaos and disorder work against incumbents, as they did in 1968 when Democrats saw their party’s popular vote fall from 61 percent to 43 percent.
Views expressed in this article are opinions of the author and do not necessarily reflect the views of The Epoch Times or ZeroHedge.
Government
Veterans Affairs Kept COVID-19 Vaccine Mandate In Place Without Evidence
Veterans Affairs Kept COVID-19 Vaccine Mandate In Place Without Evidence
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
The…
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
The U.S. Department of Veterans Affairs (VA) reviewed no data when deciding in 2023 to keep its COVID-19 vaccine mandate in place.
VA Secretary Denis McDonough said on May 1, 2023, that the end of many other federal mandates “will not impact current policies at the Department of Veterans Affairs.”
He said the mandate was remaining for VA health care personnel “to ensure the safety of veterans and our colleagues.”
Mr. McDonough did not cite any studies or other data. A VA spokesperson declined to provide any data that was reviewed when deciding not to rescind the mandate. The Epoch Times submitted a Freedom of Information Act for “all documents outlining which data was relied upon when establishing the mandate when deciding to keep the mandate in place.”
The agency searched for such data and did not find any.
“The VA does not even attempt to justify its policies with science, because it can’t,” Leslie Manookian, president and founder of the Health Freedom Defense Fund, told The Epoch Times.
“The VA just trusts that the process and cost of challenging its unfounded policies is so onerous, most people are dissuaded from even trying,” she added.
The VA’s mandate remains in place to this day.
The VA’s website claims that vaccines “help protect you from getting severe illness” and “offer good protection against most COVID-19 variants,” pointing in part to observational data from the U.S. Centers for Disease Control and Prevention (CDC) that estimate the vaccines provide poor protection against symptomatic infection and transient shielding against hospitalization.
There have also been increasing concerns among outside scientists about confirmed side effects like heart inflammation—the VA hid a safety signal it detected for the inflammation—and possible side effects such as tinnitus, which shift the benefit-risk calculus.
President Joe Biden imposed a slate of COVID-19 vaccine mandates in 2021. The VA was the first federal agency to implement a mandate.
President Biden rescinded the mandates in May 2023, citing a drop in COVID-19 cases and hospitalizations. His administration maintains the choice to require vaccines was the right one and saved lives.
“Our administration’s vaccination requirements helped ensure the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations,” the White House said.
Some experts said requiring vaccination meant many younger people were forced to get a vaccine despite the risks potentially outweighing the benefits, leaving fewer doses for older adults.
“By mandating the vaccines to younger people and those with natural immunity from having had COVID, older people in the U.S. and other countries did not have access to them, and many people might have died because of that,” Martin Kulldorff, a professor of medicine on leave from Harvard Medical School, told The Epoch Times previously.
The VA was one of just a handful of agencies to keep its mandate in place following the removal of many federal mandates.
“At this time, the vaccine requirement will remain in effect for VA health care personnel, including VA psychologists, pharmacists, social workers, nursing assistants, physical therapists, respiratory therapists, peer specialists, medical support assistants, engineers, housekeepers, and other clinical, administrative, and infrastructure support employees,” Mr. McDonough wrote to VA employees at the time.
“This also includes VA volunteers and contractors. Effectively, this means that any Veterans Health Administration (VHA) employee, volunteer, or contractor who works in VHA facilities, visits VHA facilities, or provides direct care to those we serve will still be subject to the vaccine requirement at this time,” he said. “We continue to monitor and discuss this requirement, and we will provide more information about the vaccination requirements for VA health care employees soon. As always, we will process requests for vaccination exceptions in accordance with applicable laws, regulations, and policies.”
The version of the shots cleared in the fall of 2022, and available through the fall of 2023, did not have any clinical trial data supporting them.
A new version was approved in the fall of 2023 because there were indications that the shots not only offered temporary protection but also that the level of protection was lower than what was observed during earlier stages of the pandemic.
Ms. Manookian, whose group has challenged several of the federal mandates, said that the mandate “illustrates the dangers of the administrative state and how these federal agencies have become a law unto themselves.”
Government
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International2 days ago
International2 days agoWalmart launches clever answer to Target’s new membership program
-

 International2 days ago
International2 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex





















