International
German teens went crazy for this ‘compliments’ app, and now VCs are backing its next phase
The teenage market for apps is a tough nut to crack and stay relevant in. Just ask Snapchat. Equally, teens are going through a stage in life where almost…
The teenage market for apps is a tough nut to crack and stay relevant in. Just ask Snapchat. Equally, teens are going through a stage in life where almost every social interaction seems to carry portent of some kind of other. This would explain in part why apps like SendIt, NGL, and Nocapp (some are Snapchat connected tools) took off as ways for teens to anonymously comment on each other. And AskFM would probably like us all to forget the various suicides that occurred when it was released in its initial form, back in the day. (And you thought Instagram is bad for mental health…).
Meanwhile, somehow (somehow!?) a new startup has appeared with the idea that yet another app is going to help this dumpster fire of social interactions, but let’s hear them out before jumping to conclusions.
“Slay” bills itself as a “positive social media network for teenagers”. The reason we are talking about it today is that it’s grown like a weed after launching last year in Germany, where it reached Number 1 on the German iOS App Store four days after launch. It’s now claiming to have over 250,000 registered users and claims its gaining traction in other countries including the UK, where it recently launched.
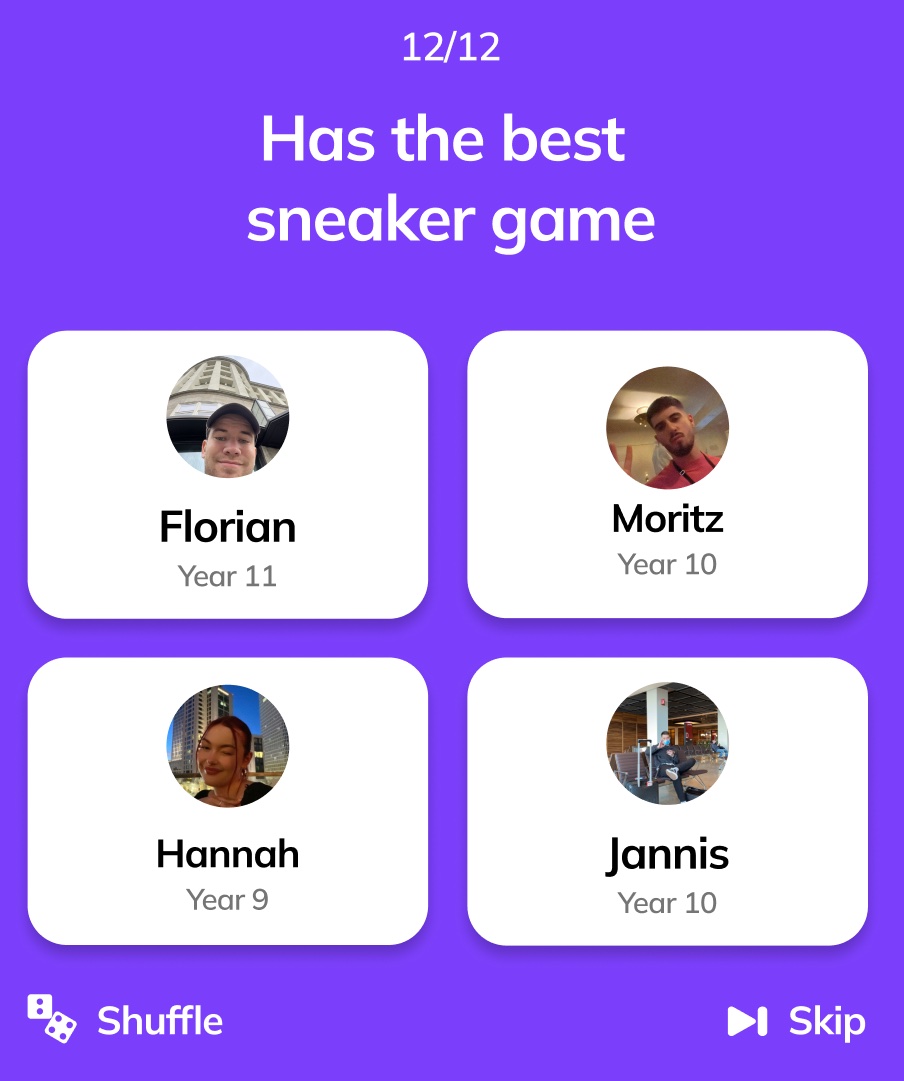
So what’s the attraction here? When users open the app it shows users 12 questions which the user can only answer by choosing another user (from their school, class or peer group) to pay an anonymous compliment to (or “slay”). For example, the app may ask a user “Who inspires me to do my best?”. They can then choose from four other users from their school to pay this ‘slay’ to. They can then view compliments from other kids, provided they answer the 12 questions when logging on. The identity of those who sent the compliment remains hidden.
This reminds me of BeReal’s mechanic, where you can only see other people’s BeReal photos by uploading your own.
And Slay is also not dissimilar to Gas, the messaging platform popular among teens for its positive spin on social media, acquired by Discord yesterday. On Gas, anonymous polling is intended to boost users’ confidence.
The other reason Slay has popped onto the TC radar, is that its growth has attracted the interest of VCs.
It’s now raised a $2.63m (€2.5m) pre-seed funding round led by Accel. Also participating was 20VC. Additional investors including Supercell Co-Founder and CEO Ilkka Paananen, Behance founder Scott Belsky, football star Mario Götze, Kevin Weil (Scribble Ventures) and musician Alex Pall (The Chainsmokers).
Slay says it is aiming to reset the teen relationship with social apps by re-balancing things away from the negative sentiments on social platforms, by normalising the giving of compliments. It also says it’s been designed with safety, content moderation and teenage mental wellbeing built in. We shall see…
Digging into the app, one can see that it’s been built very simply as a ‘compliment app’. Whether that is going to be enough to keep users coming back is hard to say. Teenager behaviour is hard to second guess. Getting zero can also send a ‘signal’, for instance.
Suffice it to say, Slay claims it will “never sell or share personal data with third parties.” Given the history of social apps, let’s see how long this lasts.
There is also no direct messaging facility, although users will be able to add links to social media profiles, so clearly they will be able message each other eventually, off-app.
Adults are supposedly not allowed to ‘join’ schools, and approximate location is asked for to suggest nearby schools. Any questions and interactions are asked by the app, not by users themselves.
SLAY was founded in 2022 by a team of three 23-year old, Berlin-based co-founders: Fabian Kamberi, Jannis Ringwald and Stefan Quernhorst. The idea was Kamberi’s, who had been building consumer apps since he was a teenager, and says he was inspired by the experiences of his siblings struggled with the negativity of social media apps during the COVID-19 pandemic.
CEO and Co-founder Kamberi, told me via email: “We see Slay in the future not only as an anonymous polling app [referring to the aforementioned Gas], but as the go-to spot for teens to rediscover social interactions in various play modes.”
“Our app is similar to Gas, and their acquisition shows a great proof of what we have built and what is in store for the future in our space. However, apps that rely solely on anonymous Q&A, for example, carry a high cyberbullying risk, which – by contrast – we prevent through our rigorous content moderation as well as specially designed gamemodes,” he added.
But the question is, why does he think a social app can improve mental health when so many social apps have not?
“We have received thousands of feedback messages from users thanking us for making them feel valued in times of fast moving, negative social media interactions,” he told me.

He said the startup could well ship new features which might create more engagement but at the same time it might bring a risk for negativity: “So we focus very much on the individual experience that each user has, aiming to make it as positive as possible.” He said the startup’s job is “content safety.”
So what’s Slay’s business model? How will it make money? Kamberi says it will likely be premium features, services or tools which users pay for: “We are currently building several exclusive, paid play modes as well as add ons, which we will release through feedback cycles with users and supported by data.”
SLAY is available in Germany, Austria, Switzerland and the United Kingdom.
Julien Bek, Principal at Accel, added via a statement: “We’re extremely impressed by the SLAY app, both in its immediate popularity among teenagers and the team’s positive goal of improving teenage mental health in the digital world. Already, the SLAY team has seen almost half its active users use it every school day.”
German teens went crazy for this ‘compliments’ app, and now VCs are backing its next phase by Mike Butcher originally published on TechCrunch
uk germany pandemic covid-19International
EyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
Ramiro Ribeiro
After six years as head of clinical development at Apellis Pharmaceuticals, Ramiro Ribeiro is joining EyePoint Pharmaceuticals as CMO.
“The…

After six years as head of clinical development at Apellis Pharmaceuticals, Ramiro Ribeiro is joining EyePoint Pharmaceuticals as CMO.
“The retinal community is relatively small, so everybody knows each other,” Ribeiro told Endpoints News in an interview. “As soon as I started to talk about EyePoint, I got really good feedback from KOLs and physicians on its scientific standards and quality of work.”
Ribeiro kicked off his career as a clinician in Brazil, earning a doctorate in stem cell therapy for retinal diseases. He previously held roles at Alcon and Ophthotech Corporation, now known as Astellas’ M&A prize Iveric Bio.
At Apellis, Ribeiro oversaw the Phase III development, filing and approval of Syfovre, the first drug for geographic atrophy secondary to age-related macular degeneration (AMD). The complement C3 inhibitor went on to make $275 million in 2023 despite reports of a rare side effect that only emerged after commercialization.
Now, Ribeiro is hoping to replicate that success with EyePoint’s lead candidate, EYP-1901 for wet AMD, which is set to enter the Phase III LUGANO trial in the second half of the year after passing a Phase II test in December.
Ribeiro told Endpoints he was optimistic about the company’s intraocular sustained-delivery tech, which he said could help address treatment burden and compliance issues seen with injectables. He also has plans to expand the EyePoint team.
“My goal is not just execution of the Phase III study — of course that’s a priority — but also looking at the pipeline and which different assets we can bring in to leverage the strength of the team that we have,” Ribeiro said.
— Ayisha Sharma
 Remco Steenbergen
Remco Steenbergen→ Sandoz CFO Colin Bond will retire on June 30 and board member Remco Steenbergen will replace him. Steenbergen, who will step down from the board when he takes over on July 1, had a 20-year career with Philips and has held the group CFO post at Deutsche Lufthansa since January 2021. Bond joined Sandoz nearly two years ago and is the former finance chief at Evotec and Vifor Pharma. Investors didn’t react warmly to Wednesday’s news as shares fell by almost 4%.
The Swiss generics and biosimilars company, which finally split from Novartis in October 2023, has also nominated FogPharma CEO Mathai Mammen to the board of directors. The ex-R&D chief at J&J will be joined by two other new faces, Swisscom chairman Michael Rechsteiner and former Unilever CFO Graeme Pitkethly.
On Monday, Sandoz said it completed its $70 million purchase of Coherus BioSciences’ Lucentis biosimilar Cimerli sooner than expected. The FDA then approved its first two biosimilars of Amgen’s denosumab the next day, in a move that could whittle away at the pharma giant’s market share for Prolia and Xgeva.
 Sean Marett
Sean Marett→ BioNTech’s chief business and commercial officer Sean Marett will retire on July 1 and will have an advisory role “until the end of the year,” the German drugmaker said in a release. Legal chief James Ryan will assume CBO responsibilities and BioNTech plans to name a new chief commercial officer by the end of the month. Marett was hired as BioNTech’s COO in 2012 after gigs at GSK, Evotec and Next Pharma, and led its commercial efforts as the Pfizer-partnered Comirnaty received the first FDA approval for a Covid-19 vaccine. BioNTech has also built a cancer portfolio that TD Cowen’s Yaron Werber described as “one of the most extensive” in biotech, from antibody-drug conjugates to CAR-T therapies.
 Chris Austin
Chris Austin→ GSK has plucked Chris Austin from Flagship and he’ll start his new gig as the pharma giant’s SVP, research technologies on April 1. After a long career at NIH in which he was director of the National Center for Advancing Translational Sciences (NCATS), Austin became CEO of Flagship’s Vesalius Therapeutics, which debuted with a $75 million Series A two years ago this week but made job cuts that affected 43% of its employees six months into the life of the company. In response to Austin’s departure, John Mendlein — who chairs the board at Sail Biomedicines and has board seats at a few other Flagship biotechs — will become chairman and interim CEO at Vesalius “later this month.”
→ BioMarin has lined up Cristin Hubbard to replace Jeff Ajer as chief commercial officer on May 20. Hubbard worked for new BioMarin chief Alexander Hardy as Genentech’s SVP, global product strategy, immunology, infectious diseases and ophthalmology, and they had been colleagues for years before Hardy was named Genentech CEO in 2019. She shifted to Roche Diagnostics as global head of partnering in 2021 and had been head of global product strategy for Roche’s pharmaceutical division since last May. Sales of the hemophilia A gene therapy Roctavian have fallen well short of expectations, but Hardy insisted in a recent investor call that BioMarin is “still very much at the early stage” in the launch.
 Pilar de la Rocha
Pilar de la Rocha→ BeiGene has promoted Pilar de la Rocha to head of Europe, global clinical operations. After 13 years in a variety of roles at Novartis, de la Rocha was named global head of global clinical operations excellence at the Brukinsa maker in the summer of 2022. A short time ago, BeiGene ended its natural killer cell therapy alliance with Shoreline Biosciences, saying that it was “a result of BeiGene’s internal prioritization decisions and does not reflect any deficit in Shoreline’s platform technology.”
 Andy Crockett
Andy Crockett→ Andy Crockett has resigned as CEO of KalVista Pharmaceuticals. Crockett had been running the company since its launch in 2011 and will hand the keys to president Ben Palleiko, who joined KalVista in 2016 as CFO. Serious safety issues ended a Phase II study of its hereditary angioedema drug KVD824, but KalVista is mounting a comeback with positive Phase III results for sebetralstat in the same indication and could compete with Takeda’s injectable Firazyr. “If approved, sebetralstat may offer a compelling treatment option for patients and their caregivers given the long-standing preference for an effective and safe oral therapy that provides rapid symptom relief for HAE attacks,” Crockett said last month.
 Steven Lo
Steven Lo→ Vaxart has tapped Steven Lo as its permanent president and CEO, while interim chief Michael Finney will stay on as chairman. Endpoints News last caught up with Lo when he became CEO at Valitor, the UC Berkeley spinout that raised a $28 million Series B round in October 2022. The ex-Zosano Pharma CEO had a handful of roles in his 13 years at Genentech before his appointments as chief commercial officer of Corcept Therapeutics and Puma Biotechnology. Andrei Floroiu resigned as Vaxart’s CEO in mid-January.
 Kartik Krishnan
Kartik Krishnan→ Kartik Krishnan has taken over for Martin Driscoll as CEO of OncoNano Medicine, and Melissa Paoloni has moved up to COO at the cancer biotech located in the Dallas-Fort Worth suburb of Southlake. The execs were colleagues at Arcus Biosciences, Gilead’s TIGIT partner: Krishnan spent two and a half years in the CMO post, while Paoloni was VP of corporate development and external alliances. In 2022, Krishnan took the CMO job at OncoNano and was just promoted to president and head of R&D last November. Paoloni came on board as OncoNano’s SVP, corporate development and strategy not long after Krishnan’s first promotion.
→ Genesis Research Group, a consultancy specializing in market access, has brought in David Miller as chairman and CEO, replacing co-founder Frank Corvino — who is transitioning to the role of vice chairman and senior advisor. Miller joins the New Jersey-based team with a number of roles under his belt from Biogen (SVP of global market access), Elan (VP of pharmacoeconomics) and GSK (VP of global health outcomes).
 Adrian Schreyer
Adrian Schreyer→ Adrian Schreyer helped build Exscientia’s AI drug discovery platform from the ground up, but he has packed his bags for Nimbus Therapeutics’ AI partner Anagenex. The new chief technology officer joined Exscientia in 2013 as head of molecular informatics and was elevated to technology chief five years later. He then held the role of VP, AI technology until January, a month before Exscientia fired CEO Andrew Hopkins.
→ Paul O’Neill has been promoted from SVP to EVP, quality & operations, specialty brands at Mallinckrodt. Before his arrival at the Irish pharma in March 2023, O’Neill was executive director of biologics operations in the second half of his 12-year career with Merck driving supply strategy for Keytruda. Mallinckrodt’s specialty brands portfolio includes its controversial Acthar Gel (a treatment for flares in a number of chronic and autoimmune indications) and the hepatorenal syndrome med Terlivaz.
 David Ford
David Ford→ Staying in Ireland, Prothena has enlisted David Ford as its first chief people officer. Ford worked in human resources at Sanofi from 2002-17 and then led the HR team at Intercept, which was sold to Italian pharma Alfasigma in late September. We recently told you that Daniel Welch, the former InterMune CEO who was a board member at Intercept for six years, will succeed Lars Ekman as Prothena’s chairman.
 Ben Stephens
Ben Stephens→ Co-founded by Sanofi R&D chief Houman Ashrafian and backed by GSK, Eli Lilly partner Sitryx stapled an additional $39 million to its Series A last fall. It has now welcomed a pair of execs: Ben Stephens (COO) had been finance director for ViaNautis Bio and Rinri Therapeutics, and Gordon Dingwall (head of clinical operations) is a Roche and AstraZeneca vet who led development operations at Mission Therapeutics. Dingwall has also served as a clinical operations leader for Shionogi and Freeline Therapeutics.
 Steve Alley
Steve Alley→ MBrace Therapeutics, an antibody-drug conjugate specialist that nabbed $85 million in Series B financing last November, has named Steve Alley as CSO. Alley spent two decades at Seagen before the $43 billion buyout by Pfizer and was the ADC maker’s executive director, translational sciences.
→ California cancer drug developer Apollomics, which has been mired in Nasdaq compliance problems nearly a year after it joined the public markets through a SPAC merger, has recruited Matthew Plunkett as CFO. Plunkett has held the same title at Nkarta as well as Imago BioSciences — leading the companies to $290 million and $155 million IPOs, respectively — and at Aeovian Pharmaceuticals since March 2022.
 Heinrich Haas
Heinrich Haas→ Co-founded by Oxford professor Adrian Hill — the co-inventor of AstraZeneca’s Covid-19 vaccine — lipid nanoparticle biotech NeoVac has brought in Heinrich Haas as chief technology officer. During his nine years at BioNTech, Haas was VP of RNA formulation and drug delivery.
 Kimberly Lee
Kimberly Lee→ New Jersey-based neuro biotech 4M Therapeutics is making its Peer Review debut by introducing Kimberly Lee as CBO. Lee was hired at Taysha Gene Therapies during its meteoric rise in 2020 and got promoted to chief corporate affairs officer in 2022. Earlier, she led corporate strategy and investor relations efforts for Lexicon Pharmaceuticals.
→ Another Peer Review newcomer, Osmol Therapeutics, has tapped former Exelixis clinical development chief Ron Weitzman as interim CMO. Weitzman only lasted seven months as medical chief of Tango Therapeutics after Marc Rudoltz had a similarly short stay in that position. Osmol is going after chemotherapy-induced peripheral neuropathy and chemotherapy-induced cognitive impairment with its lead asset OSM-0205.
→ Last August, cardiometabolic disease player NeuroBo Pharmaceuticals locked in Hyung Heon Kim as president and CEO. Now, the company is giving Marshall Woodworth the title of CFO and principal financial and accounting officer, after he served in the interim since last October. Before NeuroBo, Woodworth had a string of CFO roles at Nevakar, Braeburn Pharmaceuticals, Aerocrine and Fureix Pharmaceuticals.
 Claire Poll
Claire Poll→ Claire Poll has retired after more than 17 years as Verona Pharma’s general counsel, and the company has appointed Andrew Fisher as her successor. In his own 17-year tenure at United Therapeutics that ended in 2018, Fisher was chief strategy officer and deputy general counsel. The FDA will decide on Verona’s non-cystic fibrosis bronchiectasis candidate ensifentrine by June 26.
 Nancy Lurker
Nancy Lurker→ Alkermes won its proxy battle with Sarissa Capital Management and is tinkering with its board nearly nine months later. The newest director, Bristol Myers Squibb alum Nancy Lurker, ran EyePoint Pharmaceuticals from 2016-23 and still has a board seat there. For a brief period, Lurker was chief marketing officer for Novartis’ US subsidiary.
→ Chaired by former Celgene business development chief George Golumbeski, Shattuck Labs has expanded its board to nine members by bringing in ex-Seagen CEO Clay Siegall and Tempus CSO Kate Sasser. Siegall holds the top spots at Immunome and chairs the board at Tourmaline Bio, while Sasser came to Tempus from Genmab in 2022.
 Scott Myers
Scott Myers→ Ex-AMAG Pharmaceuticals and Rainier Therapeutics chief Scott Myers has been named chairman of the board at Convergent Therapeutics, a radiopharma player that secured a $90 million Series A last May. Former Magenta exec Steve Mahoney replaced Myers as CEO of Viridian Therapeutics a few months ago.
→ Montreal-based Find Therapeutics has elected Tony Johnson to the board of directors. Johnson is in his first year as CEO of Domain Therapeutics. He is also the former chief executive at Goldfinch Bio, the kidney disease biotech that closed its doors last year.
 Habib Dable
Habib Dable→ Former Acceleron chief Habib Dable has replaced Kala Bio CEO Mark Iwicki as chairman of the board at Aerovate Therapeutics, which is signing up patients for Phase IIb and Phase III studies of its lead drug AV-101 for pulmonary arterial hypertension. Dable joined Aerovate’s board in July and works part-time as a venture partner for RA Capital Management.
 Julie Cherrington
Julie Cherrington→ In the burgeoning world of ADCs, Elevation Oncology is developing one of its own that targets Claudin 18.2. Its board is now up to eight members with the additions of Julie Cherrington and Mirati CMO Alan Sandler. Cherrington, a venture partner at Brandon Capital Partners, also chairs the boards at Actym Therapeutics and Tolremo Therapeutics. Sandler took the CMO job at Mirati in November 2022 and will stay in that position after Bristol Myers acquired the Krazati maker.
 Patty Allen
Patty Allen→ Lonnie Moulder’s Zenas BioPharma has welcomed Patty Allen to the board of directors. Allen was a key figure in Vividion’s $2 billion sale to Bayer as the San Diego biotech’s CFO, and she’s a board member at Deciphera Pharmaceuticals, SwanBio Therapeutics and Anokion.
→ In January 2023, Y-mAbs Therapeutics cut 35% of its staff to focus on commercialization of Danyelza. This week, the company has reserved a seat on its board of directors for Nektar Therapeutics CMO Mary Tagliaferri. Tagliaferri also sits on the boards of Enzo Biochem and is a former board member of RayzeBio.
→ The ex-Biogen neurodegeneration leader at the center of Aduhelm’s controversial approval is now on the scientific advisory board at Asceneuron, a Swiss-based company focused on Alzheimer’s and Parkinson’s. Samantha Budd-Haeberlein tops the list of new SAB members, which also includes Henrik Zetterberg, Rik Ossenkoppele and Christopher van Dyck.
nasdaq covid-19 vaccine treatment fda therapy rna brazil europeInternational
Deflationary pressures in China – be careful what you wish for
Until recently, China’s decelerating inflation was welcomed by the West, as it led to lower imported prices and helped reduce inflationary pressures….

Until recently, China’s decelerating inflation was welcomed by the West, as it led to lower imported prices and helped reduce inflationary pressures. However, China’s consumer prices fell for the third consecutive month in December 2023, delaying the expected rebound in economic activity following the lifting of COVID-19 controls. For calendar year 2023, CPI growth was negligible, whilst the producer price index declined by 3.0 per cent.
China’s inflation dynamics

Chinese consumers are hindered by the weaker residential property market and high youth unemployment. Several property developers have defaulted, collectively wiping out nearly all the U.S.$155 billion worth of U.S. dollar denominated-bonds.
Meanwhile, the Shanghai Composite Index is at half of its record high, recorded in late 2007. The share prices of major developers, including Evergrande Group, Country Garden Holdings, Sunac China and Shimao Group, have declined by an average of 98 per cent over recent years. Some economists are pointing to the Japanese experience of a debt-deflation cycle in the 1990s, with economic stagnation and elevated debt levels.
Australia has certainly enjoyed the “pull-up effect” from China, particularly with the iron-ore price jumping from around U.S.$20/tonne in 2000 to an average closer to U.S.$120/tonne over the 17 years from 2007. With strong volume increases, the value of Australia’s iron ore exports has jumped 20-fold to around A$12 billion per month, accounting for approximately 35 per cent of Australia’s exports.
For context, China takes 85 per cent of Australia’s iron ore exports, whilst Australia accounts for 65 per cent of China’s iron ore imports. China’s steel industry depends on its own domestic iron ore mines for 20 per cent of its requirement, however, these are high-cost operations and need high iron ore prices to keep them in business. To reduce its dependence on Australia’s iron ore, China has increased its use of scrap metal and invested large sums of money in Africa, including the Simandou mine in Guinea, which is forecast to export 60 million tonnes of iron ore from 2028.
The Chinese housing market has historically been the source of 40 per cent of China’s steel usage. However, the recent high iron ore prices are attributable to the growth in China’s industrial and infrastructure activity, which has offset the weakness in residential construction.
Whilst this has continued to deliver supernormal profits for Australia’s major iron ore producers (and has greatly assisted the federal budget), watch out for any sustainable downturn in the iron ore price, particularly if the deflationary pressures in China continue into the medium term.
unemployment covid-19 bonds shanghai composite housing market africa chinaInternational
Four burning questions about the future of the $16.5B Novo-Catalent deal
To build or to buy? That’s a classic question for pharma boardrooms, and Novo Nordisk is going with both.
Beyond spending billions of dollars to expand…

To build or to buy? That’s a classic question for pharma boardrooms, and Novo Nordisk is going with both.
Beyond spending billions of dollars to expand its own production capacity for its weight loss drugs, the Danish drugmaker said Monday it will pay $11 billion to acquire three manufacturing plants from Catalent. It’s part of a broader $16.5 billion deal with Novo Holdings, the investment arm of the pharma’s parent group, which agreed to acquire the contract manufacturer and take it private.
It’s a big deal for all parties, with potential ripple effects across the biotech ecosystem. Here’s a look at some of the most pressing questions to watch after Monday’s announcement.
Why did Novo do this?
Novo Holdings isn’t the most obvious buyer for Catalent, particularly after last year’s on-and-off M&A interest from the serial acquirer Danaher. But the deal could benefit both Novo Holdings and Novo Nordisk.
Novo Nordisk’s biggest challenge has been simply making enough of the weight loss drug Wegovy and diabetes therapy Ozempic. On last week’s earnings call, Novo Nordisk CEO Lars Fruergaard Jørgensen said the company isn’t constrained by capital in its efforts to boost manufacturing. Rather, the main challenge is the limited amount of capabilities out there, he said.
“Most pharmaceutical companies in the world would be shopping among the same manufacturers,” he said. “There’s not an unlimited amount of machinery and people to build it.”
While Novo was already one of Catalent’s major customers, the manufacturer has been hamstrung by its own balance sheet. With roughly $5 billion in debt on its books, it’s had to juggle paying down debt with sufficiently investing in its facilities. That’s been particularly challenging in keeping pace with soaring demand for GLP-1 drugs.
Novo, on the other hand, has the balance sheet to funnel as much money as needed into the plants in Italy, Belgium, and Indiana. It’s also struggled to make enough of its popular GLP-1 drugs to meet their soaring demand, with documented shortages of both Ozempic and Wegovy.
The impact won’t be immediate. The parties expect the deal to close near the end of 2024. Novo Nordisk said it expects the three new sites to “gradually increase Novo Nordisk’s filling capacity from 2026 and onwards.”
As for the rest of Catalent — nearly 50 other sites employing thousands of workers — Novo Holdings will take control. The group previously acquired Altasciences in 2021 and Ritedose in 2022, so the Catalent deal builds on a core investing interest in biopharma services, Novo Holdings CEO Kasim Kutay told Endpoints News.
When asked about possible site closures or layoffs, Kutay said the team hasn’t thought about that.
“That’s not our track record. Our track record is to invest in quality businesses and help them grow,” he said. “There’s always stuff to do with any asset you own, but we haven’t bought this company to do some of the stuff you’re talking about.”
What does it mean for Catalent’s customers?
Until the deal closes, Catalent will operate as a standalone business. After it closes, Novo Nordisk said it will honor its customer obligations at the three sites, a spokesperson said. But they didn’t answer a question about what happens when those contracts expire.
The wrinkle is the long-term future of the three plants that Novo Nordisk is paying for. Those sites don’t exclusively pump out Wegovy, but that could be the logical long-term aim for the Danish drugmaker.
The ideal scenario is that pricing and timelines remain the same for customers, said Nicole Paulk, CEO of the gene therapy startup Siren Biotechnology.
 Nicole Paulk
Nicole Paulk“The name of the group that you’re going to send your check to is now going to be Novo Holdings instead of Catalent, but otherwise everything remains the same,” Paulk told Endpoints. “That’s the best-case scenario.”
In a worst case, Paulk said she feared the new owners could wind up closing sites or laying off Catalent groups. That could create some uncertainty for customers looking for a long-term manufacturing partner.
Are shareholders and regulators happy?
The pandemic was a wild ride for Catalent’s stock, with shares surging from about $40 to $140 and then crashing back to earth. The $63.50 share price for the takeover is a happy ending depending on the investor.
On that point, the investing giant Elliott Investment Management is satisfied. Marc Steinberg, a partner at Elliott, called the agreement “an outstanding outcome” that “clearly maximizes value for Catalent stockholders” in a statement.
Elliott helped kick off a strategic review last August that culminated in the sale agreement. Compared to Catalent’s stock price before that review started, the deal pays a nearly 40% premium.
 Alessandro Maselli
Alessandro MaselliBut this is hardly a victory lap for CEO Alessandro Maselli, who took over in July 2022 when Catalent’s stock price was north of $100. Novo’s takeover is a tacit acknowledgment that Maselli could never fully right the ship, as operational problems plagued the company throughout 2023 while it was limited by its debt.
Additional regulatory filings in the next few weeks could give insight into just how competitive the sale process was. William Blair analysts said they don’t expect a competing bidder “given the organic investments already being pursued at other leading CDMOs and the breadth and scale of Catalent’s operations.”
The Blair analysts also noted the companies likely “expect to spend some time educating relevant government agencies” about the deal, given the lengthy closing timeline. Given Novo Nordisk’s ascent — it’s now one of Europe’s most valuable companies — paired with the limited number of large contract manufacturers, antitrust regulators could be interested in taking a close look.
Are Catalent’s problems finally a thing of the past?
Catalent ran into a mix of financial and operational problems over the past year that played no small part in attracting the interest of an activist like Elliott.
Now with a deal in place, how quickly can Novo rectify those problems? Some of the challenges were driven by the demands of being a publicly traded company, like failing to meet investors’ revenue expectations or even filing earnings reports on time.
But Catalent also struggled with its business at times, with a range of manufacturing delays, inspection reports and occasionally writing down acquisitions that didn’t pan out. Novo’s deep pockets will go a long way to a turnaround, but only the future will tell if all these issues are fixed.
Kutay said his team is excited by the opportunity and was satisfied with the due diligence it did on the company.
“We believe we’re buying a strong company with a good management team and good prospects,” Kutay said. “If that wasn’t the case, I don’t think we’d be here.”
Amber Tong and Reynald Castañeda contributed reporting.
therapy pandemic europe italy-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 month ago
International1 month agoWar Delirium
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





