Government
Eli Lilly partner AbCellera finds a CBO in the face of bamlanivimab struggles; FibroGen’s next CSO walks into roxadustat rebuff
Neil Berkley
→ Covid-19 variants have marred the effectiveness of Eli Lilly and AbCellera’s bamlanivimab to such an extent that the US government stopped using the monoclonal antibody in March, followed by a similar halt for the combo treatment with…

→ Covid-19 variants have marred the effectiveness of Eli Lilly and AbCellera’s bamlanivimab to such an extent that the US government stopped using the monoclonal antibody in March, followed by a similar halt for the combo treatment with etesevimab late last month. AbCellera is nonetheless pressing onward with a new candidate for mild to moderate cases, LY-CoV1404, and this week Neil Berkley joined Carl Hansen’s squad as CBO. Berkley, a GlaxoSmithKline neuroscience veteran, makes the leap from Halozyme, starting out at the San Diego biotech as head of oncology global partnering, corporate development in 2019 and earning a promotion to VP, head of business development.
→ Skunked by an FDA adcomm’s emphatic roxadustat downvote yesterday, FibroGen picked an interesting week to break in another CSO with John Hunter getting the nod. His predecessor, Percy Carter, didn’t last a year before he scampered off to Blueprint Medicines in May to take that CSO job. Now it’s up to Hunter — the ex-chief scientist at Compugen — to dive in to this situation after a year as chief executive & scientific officer of Keyhole Therapeutics.
Unease about roxadustat’s safety prompted the Cardiovascular and Renal Drugs Advisory Committee to vote against the anemia drug by wide margins: 12-2 for patients on dialysis with anemia from chronic kidney disease, and 13-1 for non-dialysis patients.
 Joseph Romanelli
Joseph Romanelli→ RTW Investments-backed Ji Xing Pharmaceuticals has tapped Joseph Romanelli to steer the helm of the ship as CEO. Romanelli comes aboard with experience from his time at Merck, where he most recently served as president of MSD China — overseeing the country’s launches of Keytruda and Gardasil. In addition to his stint as president, Romanelli previously served as president of US human health and VP of investor relations.
 Weidong Zhong
Weidong Zhong→ Former Terns Pharmaceuticals CEO Weidong Zhong has turned up at Sciwind Biosciences USA — the US subsidiary of Hangzhou Sciwind Biosciences — as chief strategy officer and president. Prior to founding Terns, Zhong was CFO of Nightstar Therapeutics, which was later acquired by Biogen in a buyout that has turned into a major headache. Zhong was the global head of antiviral research at Novartis and the senior director of biology at Gilead. In August, Senthil Sundaram took over as CEO of Terns, while Zhong settled into the roles of CSO and chairman, but only fleetingly.
 Howard Liang
Howard Liang→ Tessera’s last big batch of execs rolled in this spring with ex-bluebird CMO David Davidson leading the pack. This batch is even bigger: CEO Geoff von Maltzahn has pulled together six more teammates while promoting another at the Flagship-backed gene writing biotech, headlined by president and CFO Howard Liang, who was CFO and chief strategy officer during his six-year run at BeiGene. Our Nicole DeFeudis spoke with him before Wednesday’s announcement.
 Cecilia Cotta-Ramusino
Cecilia Cotta-RamusinoHere are the other folks donning the Tessera jersey: Madhusudan Peshwa (CTO for cell therapy) had been CTO of Mana Therapeutics and was CTO and global head of R&D for GE Healthcare’s cell and gene therapies business; Bill Querbes (SVP, therapeutic discovery & translational sciences) is an Alnylam vet who comes to Tessera after his time as Avrobio’s VP and Fabry program lead; Cecilia Cotta-Ramusino (SVP, platform development) was employee no. 1 at insitro as head of functional genomics after her four years with Editas Medicine; Vikram Ranade tacks on SVP to his title after joining Tessera last year as head of corporate development; David Pollard (head of bioprocess) logged more than two decades at Merck and is the former executive director of process sciences at Kite; and finally, Steve Garbacz (head of finance) made stops at Biogen, Epizyme, and Spero before his most recent gig as VP, finance and corporate controller for Anika Therapeutics.
 Megan Baierlein
Megan Baierlein→ There’s no off button for the Audentes purge as Megan Baierlein sets up shop at T cell receptor (TCR) biotech T-knife Therapeutics as COO. Baierlein was likewise the COO at Audentes, now Astellas Gene Therapies and rattled by the deaths of two patients that could set back approval of its gene therapy at least until the middle of 2022. She’s made other stops along the way at Genentech, BioMarin, and Ultragenyx before this new gig at Versant-backed T-knife, which raked in a $78 million Series A round almost a year ago and named Thomas Soloway CEO in January.
Multiple Audentes staffers have since moved on to greener pastures in the last several months. With their new companies in parentheses, that list includes CMO Edward Conner (Locanabio), CCO Eric Mosbrooker (Cognoa), SVP of technical operations Donald Wuchterl (now Baierlein’s colleague at T-knife), and SVP of human resources Mary Newman (Taysha).
→ When last we saw Thomas Lars Andresen, he co-founded and was CSO of Torque Therapeutics, which Flagship then fused together with Cogen to form Repertoire Immune Medicines last year. Starting Aug. 1, Andersen becomes CEO and a board member at T-Cypher Bio, Oxford’s TCR biotech that broke free from Orbit Discovery in February. Andresen also founded medical device outfit Nanovi and MonTa Biosciences, both located in Denmark.
 Bryan Dechairo
Bryan Dechairo→ Elementary, my dear Peer Reviewers: Bryan Dechairo is succeeding co-founder Rahul Dhanda as president and CEO of Feng Zhang’s diagnostic upstart Sherlock Biosciences, which launched in 2019. A Pfizer vet in molecular medicine, Dechairo was previously Myriad Genetics’ SVP of clinical development, a position he had held since 2017. Dhanda remains a board member until the end of the year and then he will become a strategic advisor in 2022 for Sherlock, so named for its CRISPR-based technology platform (Specific High-sensitivity Enzymatic Reporter unLOCKing).
→ After launching early last month with a $12.8 million seed round led by Takeda Ventures and the Dementia Discovery Fund, Transine Therapeutics has plucked up Jan Thirkettle as CEO. Thirkettle is no stranger to the executive seat, having previously served as CEO and chief development officer at Freeline Therapeutics. Prior to his career at Freeline, Thirkettle spent 18 years at GSK, including roles spanning from discovery to manufacturing.
→ Starting July 1, Philip Kantoff took on the CEO role at Cambridge, MA-based radiopharmaceutical company Convergent Therapeutics — with its lead candidate CONV 01-α being developed to treat prostate cancer. Kantoff had been chairman of the Department of Medicine at Memorial Sloan Kettering since 2015, and was the longtime director of the Lank Center for Genitourinary Oncology at Dana-Farber.
 Rebecca Chambers
Rebecca Chambers→ Genomic diagnostics player Veracyte is becoming another Peer Review mainstay, with Rebecca Chambers stepping up to the plate as EVP and CFO just days after CBO Rob Brainin and chief information officer Bill Zondler were added to new CEO Marc Stapley’s team. Chambers just completed a run as CFO at Outset Medical and was VP, financial planning & analysis to close out nearly seven years at Illumina from 2012-19.
→ Jazzed about the obe-cel data it presented to the European Hematology Association for indolent B cell non-Hodgkin lymphoma, London-based Autolus has ushered in Edgar Braendle as chief development officer. The Novartis oncology vet just had a year-long stay as CMO and global head of development with Sumitomo Dainippon Pharma Oncology. Autolus named ex-Syncona chief Martin Murphy chairman in April after trimming 20% of its workforce to kick off 2021.
 Kelly Gold
Kelly Gold→ Kelly Gold has the Midas touch at Camp4 Therapeutics with her promotion to CBO and SVP of finance, while Michelle Gates steps in as SVP and chief people officer. Gold has served as Camp4’s director, corporate development & finance and VP, corporate development since punching her ticket out of Biogen in 2017. Gates had devoted her last 16 years to a series of human resources capacities at Akamai Technologies before this new position at CEO Josh Mandel-Brehm’s regRNA biotech.
One more Camp4 nugget: Mass General Brigham‘s chief academic officer Ravi Thadhani takes a seat on the board of directors.
→ Making its debut in May with Alexion co-founder Stephen Squinto as CEO and $40 million worth of fresh Series A funding at its disposal, Gennao Bio has locked in Chris Duke as COO. Duke used to be in charge of operations for Amicus’ Japanese affiliate and led the commercialization in Tokyo for its Fabry disease drug Galafold. He also has COO experience from Advaxis and was NPS Pharma’s executive director of international commercial operations. Additionally, ex-Arch Oncology interim CEO and ubiquitous Peer Review presence Julie Hambleton is now part of Gennao Bio’s board of directors.
 Katherine Ruffner
Katherine Ruffner→ Once known as Trovagene, San Diego-based Cardiff Oncology has bulked up its C-suite with CMO Katherine Ruffner and CFO James Levine. Ruffner has worked in oncology development with Pfizer, Biogen and Amgen, and she comes to the onvansertib biotech from ALX Oncology, where she was VP of clinical development. Since late 2018, Levine had been CFO with Cidara Therapeutics, helping strike a deal this spring with J&J in which the pharma giant staked up to $780 million on Cidara’s influenza drugs.
 Gbola Amusa
Gbola Amusa→ Before all the kids were doing it, Chardan chose the SPAC route with Chardan Healthcare Acquisition and reverse-merged with BiomX in 2019. Gbola Amusa was at the forefront of that effort, and this week Chardan promoted him to CSO. Amusa, who will still be listed as a partner, spent the last seven years as Chardan’s head of healthcare research.
→ Co-founded by Luigi Naldini last year, Milan gene therapy player Genespire has tapped Philippe Mauberna as CFO. Mauberna spent the last eight years as CFO of Nanobiotix, which just presented data at ASCO for its hafnium oxide nanoparticle drug NBTXR3, and he took on the same title previously at Astellas Pharma France. Mauberna’s association with Astellas goes back to his days as the company’s senior director, EMEA, market planning and commercial effectiveness in the UK.
 Sean Grant
Sean Grant→ Overhauling its focus from gene therapy to kidney disease and receiving a tepid response on Nasdaq, Vera Therapeutics (formerly Trucode Gene Repair) has turned to Sean Grant to be CFO. Grant, a one-time Citigroup exec in healthcare investment banking, swings over to Vera after a year as VP of corporate strategy and business development for CareDx.
→ Corcept Therapeutics has enlisted Gilead alum Amy Flood as chief human resources and communications officer. Flood gave 21 years to Gilead and had been serving as SVP, public affairs for Dan O’Day’s bunch. Her appointment comes several months after Corcept — the first biotech to secure an FDA approval for a Cushing’s syndrome drug with Korlym in 2012 — made a couple of leadership readjustments by slotting ex-CFO Charles Robb into the CBO role, paving the way for current CFO Atabak Mokari.
 Anna Quattropani
Anna Quattropani→ With ex-Dermira CEO Andrew Hotchkiss now at the helm, Danish PCSK9 biotech Draupnir Bio’s latest hire is chief discovery officer Anna Quattropani. She had a long association with Serono (later Merck Serono) in medicinal chemistry and then shifted to its spinoff Asceneuron, spending the last eight years there and rising to EVP and head of non-clinical development and medicinal chemistry.
→ Brooklyn ImmunoTherapeutics, the IL-2 biotech in a Phase IIa trial with IRX-2 for head and neck cancer, nets another exec with chief administrative officer Jay Sial after CSO Kevin D’Amour’s appointment in June. Sial just completed a brief stint as CFO of Aspen Neuroscience, now under the leadership of CEO Damien McDevitt.
→ Biosimulation-focused Certara has handed the top human resources job to Nicolette Sherman, who was also the chief human resources officer at Oyster Point Pharma and held HR and leadership development posts during her 11 years with Sanofi. Additionally, Certara has recruited former McKinsey director Nancy Killefer and ex-Editas Medicine CEO Cynthia Collins to its board of directors.
 Timothy Zheng
Timothy Zheng→ Cygnal put out word on Twitter that Timothy Zheng has climbed aboard as SVP of biology. Previously, Zheng was a research fellow and executive director of immune modulation at Boehringer Ingelheim and also spent 17 years at Biogen. The Pearl Huang-led Flagship biotech exploring the exoneural biology field later tweeted that Arrakis co-founder and CSO Jennifer Petter has joined the scientific advisory board.
→ Anna Diaz Triola has signed on as chief commercial officer of AstraZeneca’s antibacterial spinout Entasis Therapeutics. The ex-Biogen product manager and associate director grabs this opportunity after consecutive VP of marketing stints at Flexion Therapeutics and Summit Therapeutics. Earlier, Triola closed out her six years at Cubist Pharmaceuticals as head of patient engagement & advocacy.
 Michael Brooks
Michael Brooks→ Syneos Health, the CRO located in the Research Triangle of North Carolina, has appointed Michael Brooks as its first chief development officer. Brooks, who took on a basket full of roles during his days at PPD from 1999-2014, is the ex-president & global head of clinical development & commercialization services for LabCorp (Covance).
→ Narcan maker Opiant Pharmaceuticals has named Matthew Ruth as its CCO. Prior to his new role, Ruth was COO for medtech company RightCare Solutions. Before that, Ruth was VP of Azur Pharma and Avanir Pharmaceuticals and held a variety of roles at Allergan.
 Sharon Morriss
Sharon Morriss→ Sharon Morriss has been named SVP of clinical development at Austin-based orphan drug biotech Lung Therapeutics, which had its most recent round of funding two years ago. The Shire vet jumps on board from Cedric Francois’ crew at Apellis, leaving her mark on the rare disease and ophthalmology clinical portfolio as SVP, clinical development operations. In May, Apellis’ paroxysmal nocturnal hemoglobinuria drug pegcetacoplan was approved by the FDA, now known as Empaveli.
→ Tavanta Therapeutics has made some changes to its leadership team with the appointment of Andreas Maetzel as CMO and the promotion of Elizabeth Manning Duus to VP of clinical development. Maetzel joins the company after a stint as CMO of KalVista Pharmaceuticals and senior global medical affairs positions with BioCryst Pharmaceuticals and Cornerstone Biopharma. Meanwhile, Duus came to Tavanta in January 2019 as executive director, clinical development after serving at Helsinn Therapeutics and Akros Pharma.
→ Gaithersburg, MD-based NexImmune threw its hat into this year’s IPO ring with a $110 million haul, and the biotech has sewn up two new hires with SVP of translational science Jack Ragheb and head of business development Matt Schiller. Ragheb hails from Eli Lilly, where he was senior medical fellow for immunology and co-chaired the immunogenicity/immunosafety working group. Schiller’s run with EMD Serono began in 2015, serving as director, global licensing & business development, immunology.
→ Developing an AAV-based gene therapy for BAG3-associated dilated cardiomyopathy (DCM), Renovacor materializes with another new exec after adding CMO Marc Semigran, with Jiwen Zhang heading to the biotech as SVP, regulatory affairs and quality assurance. Zhang, who worked in regulatory affairs at such companies as Merck, Wyeth, Sanofi, GE Healthcare and Tmunity, had been VP, head of regulatory affairs at Passage Bio since 2019.
 Steven Neben
Steven Neben→ Mohammad El-Kalay’s June appointment as head of CMC was merely a warmup for the trio of incoming staffers at BeiGene partner Shoreline Biosciences. Boyan Litchev (SVP and head of global clinical development) has been around the block with leadership roles at Shire, Akcea and Halozyme, and in February 2020 he became head of clinical development, oncology for Poseida. Steven Neben (VP, alliance and project management) is an AnaptysBio vet who just completed a nine-year run at Regulus Therapeutics. And Bjorn Dahle (VP, smart manufacturing) is the ex-CEO of Inspire Solutions and VersaCall Technologies.
→ Shortly after The Column Group helped co-lead Circle Pharma’s $66 million Series C round, the South San Francisco macrocycle drug developer has now brought in Evelyn Wang as its VP of translational medicine. Wang, who was once the director of translational research at BioMarin, joined Exelixis in 2016 and held the role of executive director, translational medicine.
 Gayle Gironda
Gayle Gironda→ Boston-based Inozyme has reeled in Gayle Gironda as SVP of human resources. Gironda comes aboard after most recently serving as VP, human resources, global hematology and global market access at Bristol Myers Squibb. Before her role at BMS, Gironda was with Celgene and Alexion.
→ Personal Genome Diagnostics (PGDx) has welcomed back Mark Sausen to the team as VP, technology innovation. Sausen formerly served as the Baltimore-based company’s VP, research and development for six years before hopping over to a stint at Bristol Myers, where he was scientific director, clinical genetics and genomics.
→ Snapdragon Chemistry has enlisted Hui Fang as the company’s director of attribute sciences and quality control. Fang hops aboard after serving as associate director, CMC analytical development at Akebia Therapeutics. Prior to that role, Fang was with Eisai.
 Dieter Weinand
Dieter Weinand→ Former Bayer CEO Dieter Weinand is adding yet another executive chairman role to his résumé with his appointment to the board of directors at Essenlix. Currently, Weinand is the chairman of the board of Replimmune and executive chairman of ZielBioPharma. Weinand’s career has spanned stints at Pfizer, Bristol Myers and Sanofi.
→ T cell-based immunotherapy biotech Virion Therapeutics out of Philadelphia has elected Michelle Berrey and Bill Bradford to the board of directors. Berrey’s tenure as Intercept’s CMO and president of R&D is in its early stages, while Bradford co-founded and was CMO for Indalo Therapeutics.
 Jennifer Wellman
Jennifer Wellman→ Atsena Therapeutics, the Research Triangle gene therapy outfit founded by Shannon and Sanford Boye that bagged a $55 million Series A in December, has made room for Spark co-founder Jennifer Wellman on the board of directors. Wellman is now the COO at Manny Simons-led Akouos.
→ Immuneering, the oncology biotech where you can now find Biren Amin as CFO after offering his oncology takes at Jefferies, has opened up a seat for Ann Berman on the board of directors. Berman, the retired CFO and VP of finance at Harvard, has also been on the board of Loews since 2006.
→ New York-based Xalud Therapeutics has added some new faces to its strategic advisory board. The fresh recruits include: Michael Ehlers (CSO and venture partner at Apple Tree Partners); Mace Rothenberg (former CMO at Pfizer); Tarek Samad (SVP and global head of research at Lundbeck); and Matt Walker (SVP, head of industrial operations for GSK Vaccines).
 John Davis
John Davis→ When John Davis ultimately leaves Magenta Therapeutics, he has his next role lined up as a member of the board of directors at Third Rock’s immunometabolism biotech Rheos Medicines. In late May, Davis resigned as Magenta’s CMO and head of R&D, stepping aside “no later than July 30,” according to an SEC filing.
→ Michael Hayden is now a non-executive director at Oxford Biomedica. Hayden, the CEO of Forbion-backed neuro startup Prilenia Therapeutics, was the president of global R&D and CSO at Teva from 2012-17.
 Brendan Delaney
Brendan Delaney→ Brendan Delaney has been added to the board of directors at New York-based BeyondSpring. Delaney, the chief commercial officer of Immunomedics when Gilead made a $21 billion deal for the company, is in his first year as CCO at Constellation Pharmaceuticals.
→ Akero Therapeutics — who’s working on an old Amgen drug to treat NASH — has pulled in Judy Chou to its board of directors. Chou currently serves as president and CEO of AltruBio and formerly served as SVP and global head of biotech at Bayer. Earlier in her career, Chou held pharmaceutical operations and manufacturing roles at Pfizer and Tanvex Biopharma.
→ Out in the Rockies, Clovis Oncology is letting in Ronit Simantov as a member of the board of directors. The ex-VP of oncology global medical affairs at Pfizer, Simantov has been Gamida Cell’s CMO since July 2017.
nasdaq covid-19 us government treatment fda genome therapy deaths gold european uk france china
Government
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
Authored by Jack Phillips via The Epoch Times (emphasis ours),
A…

Authored by Jack Phillips via The Epoch Times (emphasis ours),
A U.S. Centers for Disease Control (CDC) paper released Thursday found that thousands of young children have been taken to the emergency room over the past several years after taking the very common sleep-aid supplement melatonin.
The agency said that melatonin, which can come in gummies that are meant for adults, was implicated in about 7 percent of all emergency room visits for young children and infants “for unsupervised medication ingestions,” adding that many incidents were linked to the ingestion of gummy formulations that were flavored. Those incidents occurred between the years 2019 and 2022.
Melatonin is a hormone produced by the human body to regulate its sleep cycle. Supplements, which are sold in a number of different formulas, are generally taken before falling asleep and are popular among people suffering from insomnia, jet lag, chronic pain, or other problems.
The supplement isn’t regulated by the U.S. Food and Drug Administration and does not require child-resistant packaging. However, a number of supplement companies include caps or lids that are difficult for children to open.
The CDC report said that a significant number of melatonin-ingestion cases among young children were due to the children opening bottles that had not been properly closed or were within their reach. Thursday’s report, the agency said, “highlights the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight,” including melatonin.
The approximately 11,000 emergency department visits for unsupervised melatonin ingestions by infants and young children during 2019–2022 highlight the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight.
The CDC notes that melatonin use among Americans has increased five-fold over the past 25 years or so. That has coincided with a 530 percent increase in poison center calls for melatonin exposures to children between 2012 and 2021, it said, as well as a 420 percent increase in emergency visits for unsupervised melatonin ingestion by young children or infants between 2009 and 2020.
Some health officials advise that children under the age of 3 should avoid taking melatonin unless a doctor says otherwise. Side effects include drowsiness, headaches, agitation, dizziness, and bed wetting.
Other symptoms of too much melatonin include nausea, diarrhea, joint pain, anxiety, and irritability. The supplement can also impact blood pressure.
However, there is no established threshold for a melatonin overdose, officials have said. Most adult melatonin supplements contain a maximum of 10 milligrams of melatonin per serving, and some contain less.
Many people can tolerate even relatively large doses of melatonin without significant harm, officials say. But there is no antidote for an overdose. In cases of a child accidentally ingesting melatonin, doctors often ask a reliable adult to monitor them at home.
Dr. Cora Collette Breuner, with the Seattle Children’s Hospital at the University of Washington, told CNN that parents should speak with a doctor before giving their children the supplement.
“I also tell families, this is not something your child should take forever. Nobody knows what the long-term effects of taking this is on your child’s growth and development,” she told the outlet. “Taking away blue-light-emitting smartphones, tablets, laptops, and television at least two hours before bed will keep melatonin production humming along, as will reading or listening to bedtime stories in a softly lit room, taking a warm bath, or doing light stretches.”
In 2022, researchers found that in 2021, U.S. poison control centers received more than 52,000 calls about children consuming worrisome amounts of the dietary supplement. That’s a six-fold increase from about a decade earlier. Most such calls are about young children who accidentally got into bottles of melatonin, some of which come in the form of gummies for kids, the report said.
Dr. Karima Lelak, an emergency physician at Children’s Hospital of Michigan and the lead author of the study published in 2022 by the CDC, found that in about 83 percent of those calls, the children did not show any symptoms.
However, other children had vomiting, altered breathing, or other symptoms. Over the 10 years studied, more than 4,000 children were hospitalized, five were put on machines to help them breathe, and two children under the age of two died. Most of the hospitalized children were teenagers, and many of those ingestions were thought to be suicide attempts.
Those researchers also suggested that COVID-19 lockdowns and virtual learning forced more children to be at home all day, meaning there were more opportunities for kids to access melatonin. Also, those restrictions may have caused sleep-disrupting stress and anxiety, leading more families to consider melatonin, they suggested.
The Associated Press contributed to this report.
International
Red Candle In The Wind
Red Candle In The Wind
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by…
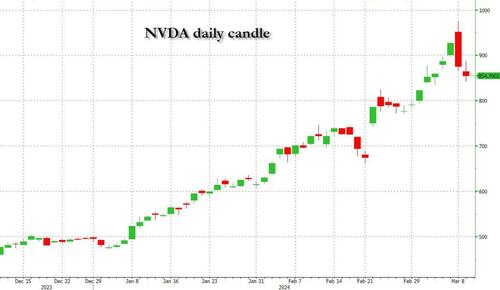
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by printing at 275,000 against a consensus call of 200,000. We say superficially, because the downward revisions to prior months totalled 167,000 for December and January, taking the total change in employed persons well below the implied forecast, and helping the unemployment rate to pop two-ticks to 3.9%. The U6 underemployment rate also rose from 7.2% to 7.3%, while average hourly earnings growth fell to 0.2% m-o-m and average weekly hours worked languished at 34.3, equalling pre-pandemic lows.
Undeterred by the devil in the detail, the algos sprang into action once exchanges opened. Market darling NVIDIA hit a new intraday high of $974 before (presumably) the humans took over and sold the stock down more than 10% to close at $875.28. If our suspicions are correct that it was the AIs buying before the humans started selling (no doubt triggering trailing stops on the way down), the irony is not lost on us.
The 1-day chart for NVIDIA now makes for interesting viewing, because the red candle posted on Friday presents quite a strong bearish engulfing signal. Volume traded on the day was almost double the 15-day simple moving average, and similar price action is observable on the 1-day charts for both Intel and AMD. Regular readers will be aware that we have expressed incredulity in the past about the durability the AI thematic melt-up, so it will be interesting to see whether Friday’s sell off is just a profit-taking blip, or a genuine trend reversal.
AI equities aside, this week ought to be important for markets because the BTFP program expires today. That means that the Fed will no longer be loaning cash to the banking system in exchange for collateral pledged at-par. The KBW Regional Banking index has so far taken this in its stride and is trading 30% above the lows established during the mini banking crisis of this time last year, but the Fed’s liquidity facility was effectively an exercise in can-kicking that makes regional banks a sector of the market worth paying attention to in the weeks ahead. Even here in Sydney, regulators are warning of external risks posed to the banking sector from scheduled refinancing of commercial real estate loans following sharp falls in valuations.
Markets are sending signals in other sectors, too. Gold closed at a new record-high of $2178/oz on Friday after trading above $2200/oz briefly. Gold has been going ballistic since the Friday before last, posting gains even on days where 2-year Treasury yields have risen. Gold bugs are buying as real yields fall from the October highs and inflation breakevens creep higher. This is particularly interesting as gold ETFs have been recording net outflows; suggesting that price gains aren’t being driven by a retail pile-in. Are gold buyers now betting on a stagflationary outcome where the Fed cuts without inflation being anchored at the 2% target? The price action around the US CPI release tomorrow ought to be illuminating.
Leaving the day-to-day movements to one side, we are also seeing further signs of structural change at the macro level. The UK budget last week included a provision for the creation of a British ISA. That is, an Individual Savings Account that provides tax breaks to savers who invest their money in the stock of British companies. This follows moves last year to encourage pension funds to head up the risk curve by allocating 5% of their capital to unlisted investments.
As a Hail Mary option for a government cruising toward an electoral drubbing it’s a curious choice, but it’s worth highlighting as cash-strapped governments increasingly see private savings pools as a funding solution for their spending priorities.
Of course, the UK is not alone in making creeping moves towards financial repression. In contrast to announcements today of increased trade liberalisation, Australian Treasurer Jim Chalmers has in the recent past flagged his interest in tapping private pension savings to fund state spending priorities, including defence, public housing and renewable energy projects. Both the UK and Australia appear intent on finding ways to open up the lungs of their economies, but government wants more say in directing private capital flows for state goals.
So, how far is the blurring of the lines between free markets and state planning likely to go? Given the immense and varied budgetary (and security) pressures that governments are facing, could we see a re-up of WWII-era Victory bonds, where private investors are encouraged to do their patriotic duty by directly financing government at negative real rates?
That would really light a fire under the gold market.
Government
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19…

Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19 vaccination was a mistake due to ethical and other concerns, a top government doctor warned Dr. Anthony Fauci after Dr. Fauci promoted mass vaccination.
“Coercing or forcing people to take a vaccine can have negative consequences from a biological, sociological, psychological, economical, and ethical standpoint and is not worth the cost even if the vaccine is 100% safe,” Dr. Matthew Memoli, director of the Laboratory of Infectious Diseases clinical studies unit at the U.S. National Institute of Allergy and Infectious Diseases (NIAID), told Dr. Fauci in an email.
“A more prudent approach that considers these issues would be to focus our efforts on those at high risk of severe disease and death, such as the elderly and obese, and do not push vaccination on the young and healthy any further.”
Employing that strategy would help prevent loss of public trust and political capital, Dr. Memoli said.
The email was sent on July 30, 2021, after Dr. Fauci, director of the NIAID, claimed that communities would be safer if more people received one of the COVID-19 vaccines and that mass vaccination would lead to the end of the COVID-19 pandemic.
“We’re on a really good track now to really crush this outbreak, and the more people we get vaccinated, the more assuredness that we’re going to have that we’re going to be able to do that,” Dr. Fauci said on CNN the month prior.
Dr. Memoli, who has studied influenza vaccination for years, disagreed, telling Dr. Fauci that research in the field has indicated yearly shots sometimes drive the evolution of influenza.
Vaccinating people who have not been infected with COVID-19, he said, could potentially impact the evolution of the virus that causes COVID-19 in unexpected ways.
“At best what we are doing with mandated mass vaccination does nothing and the variants emerge evading immunity anyway as they would have without the vaccine,” Dr. Memoli wrote. “At worst it drives evolution of the virus in a way that is different from nature and possibly detrimental, prolonging the pandemic or causing more morbidity and mortality than it should.”
The vaccination strategy was flawed because it relied on a single antigen, introducing immunity that only lasted for a certain period of time, Dr. Memoli said. When the immunity weakened, the virus was given an opportunity to evolve.
Some other experts, including virologist Geert Vanden Bossche, have offered similar views. Others in the scientific community, such as U.S. Centers for Disease Control and Prevention scientists, say vaccination prevents virus evolution, though the agency has acknowledged it doesn’t have records supporting its position.
Other Messages
Dr. Memoli sent the email to Dr. Fauci and two other top NIAID officials, Drs. Hugh Auchincloss and Clifford Lane. The message was first reported by the Wall Street Journal, though the publication did not publish the message. The Epoch Times obtained the email and 199 other pages of Dr. Memoli’s emails through a Freedom of Information Act request. There were no indications that Dr. Fauci ever responded to Dr. Memoli.
Later in 2021, the NIAID’s parent agency, the U.S. National Institutes of Health (NIH), and all other federal government agencies began requiring COVID-19 vaccination, under direction from President Joe Biden.
In other messages, Dr. Memoli said the mandates were unethical and that he was hopeful legal cases brought against the mandates would ultimately let people “make their own healthcare decisions.”
“I am certainly doing everything in my power to influence that,” he wrote on Nov. 2, 2021, to an unknown recipient. Dr. Memoli also disclosed that both he and his wife had applied for exemptions from the mandates imposed by the NIH and his wife’s employer. While her request had been granted, his had not as of yet, Dr. Memoli said. It’s not clear if it ever was.
According to Dr. Memoli, officials had not gone over the bioethics of the mandates. He wrote to the NIH’s Department of Bioethics, pointing out that the protection from the vaccines waned over time, that the shots can cause serious health issues such as myocarditis, or heart inflammation, and that vaccinated people were just as likely to spread COVID-19 as unvaccinated people.
He cited multiple studies in his emails, including one that found a resurgence of COVID-19 cases in a California health care system despite a high rate of vaccination and another that showed transmission rates were similar among the vaccinated and unvaccinated.
Dr. Memoli said he was “particularly interested in the bioethics of a mandate when the vaccine doesn’t have the ability to stop spread of the disease, which is the purpose of the mandate.”
The message led to Dr. Memoli speaking during an NIH event in December 2021, several weeks after he went public with his concerns about mandating vaccines.
“Vaccine mandates should be rare and considered only with a strong justification,” Dr. Memoli said in the debate. He suggested that the justification was not there for COVID-19 vaccines, given their fleeting effectiveness.
Julie Ledgerwood, another NIAID official who also spoke at the event, said that the vaccines were highly effective and that the side effects that had been detected were not significant. She did acknowledge that vaccinated people needed boosters after a period of time.
The NIH, and many other government agencies, removed their mandates in 2023 with the end of the COVID-19 public health emergency.
A request for comment from Dr. Fauci was not returned. Dr. Memoli told The Epoch Times in an email he was “happy to answer any questions you have” but that he needed clearance from the NIAID’s media office. That office then refused to give clearance.
Dr. Jay Bhattacharya, a professor of health policy at Stanford University, said that Dr. Memoli showed bravery when he warned Dr. Fauci against mandates.
“Those mandates have done more to demolish public trust in public health than any single action by public health officials in my professional career, including diminishing public trust in all vaccines.” Dr. Bhattacharya, a frequent critic of the U.S. response to COVID-19, told The Epoch Times via email. “It was risky for Dr. Memoli to speak publicly since he works at the NIH, and the culture of the NIH punishes those who cross powerful scientific bureaucrats like Dr. Fauci or his former boss, Dr. Francis Collins.”
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International4 days ago
International4 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















