International
Building T Cell Receptor Therapies with Generative AI
Michelle Teng, CEO and co-founder of Etcembly and co-founder of SynaptixBio, spoke with Inside Precision Medicine’s senior editor Helen Albert about her…

Immunotherapies such as checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapies have revolutionized the field of oncology over the last few years, but they are just the beginning. A new wave of immunotherapies is now hitting the clinic.
In 2022, Immunocore’s tebentafusp (Kimmtrak) was the first TCR anti-cancer therapy to receive FDA approval. While most companies developing TCR therapies are using a method called phage display to design new treatments, ex-Immunocore scientist Michelle Teng and colleagues at Etcembly have developed a method of using generative AI to discover and design a range of different TCRs to fight cancer and other conditions. They have already developed a candidate bispecific T cell engager therapy, to target a cancer antigen known as PRAME, in less than a year.
Teng, CEO and co-founder of Etcembly, is an entrepreneur as well as an experienced scientist with a background in T cell immunotherapy research. A few months after founding Etcembly in 2020 she also co-founded rare disease biotech SynaptixBio, where she retains a position as CSO, to try and find a cure for her daughter Sofia who has a rare neurodegenerative condition called TUBB4a Leukodystrophy.
Inside Precision Medicine senior editor Helen Albert spoke to Teng about her work, her inspirations and what she hopes to achieve at Etcembly and SynaptixBio.
Q: What inspired you to become a scientist?

Chief Executive Officer
Etcembly
Michelle Teng: I’ve always been driven by curiosity about how things work, where they’ve come from and how they come into being. That innate sense of curiosity, and my relentless drive to find the answers to questions moved me into science. I was at an intersection at one point in my academic life where I was considering being a lawyer. My parents thought I had the aptitude for it, because I was also very analytical and logical in my thinking. It could have gone both ways to be honest.
I did my first degree at Imperial College London in biochemistry and that consolidated and crystallized my desire to become a scientist. Having that great nurturing educational environment really helped develop that part of me.
Q: What made you decide to work in industry?
MT: I think a lot of it had to do in my postdoc supervisor at the Wellcome Sanger Institute. He’s no stranger to the field of biotech. His name is John McCafferty and he’s got this great entrepreneurial spirit that you didn’t see in many scientists back in the day. He certainly inspired me to translate whatever I was doing into a business, or at least encouraged me to think that way. He said, “Well, if you really want to be a biotech entrepreneur one day, try industry for a couple of years. If it doesn’t work, you can always return to academia.” So, I took his advice, and that’s why I ended up working at MedImmune, which is now a subsidiary of AstraZeneca.
Q: What did you learn from working as an industry scientist?
MT: I did enjoy it, but I also decided what ‘good’ looked like for me. I didn’t want to be in a large organization where you were just a tiny cog in the wheel and you don’t really understand the bigger picture. I wanted to be part of something small to give me the exposure to what biotech is about.
When I transitioned to Immunocore, that helped cement the direction of my career. I never thought going into industry meant I wanted to spin out a company one day. I was actually quite content to be tinkering in the lab for the rest of my life. At least, that was my romantic view of myself! At some point I realized the work I had done at Immunocore could be done better. If I could find the right resources and funding then I would at least have a decent shot at it. So that’s what I decided to do and why I ended up being a founder.
Q: What was the inspiration to start two biotech companies?
MT: I founded SynaptixBio and Etcembly around a couple of months apart. My daughter was diagnosed with a rare neurological disease [TUBB4a Leukodystrophy] that is life limiting. I initially started a foundation to raise awareness and raise money for research. But I realized that the money that I raised is never going to be enough to take something into the clinic. That’s why the company SynaptixBio was formed, we decided that we had to set up a commercial entity and fund it properly.
So far, everything has actually gone very well. We have a lead candidate; we are manufacturing the drugs. Everything that we have within our control is actually moving according to plan. The regulatory side has been met with lots of obstacles. But overall, from the inception of the company we have met all our milestones with very little delay.
Etcembly, in a way, is a form of escapism. If you found a company to treat your daughter, you need something else to distract your mind, to solve a different problem in a different space. I knew I could apply myself in different ways and that’s what I wanted to do. It is a lot of work, but it’s a lot of fun as well. I don’t regret a single minute of it.
Q: What was the transition like from being a senior scientist at Immunocore to a founder of two biotech companies?
MT: I was a group leader at Immunocore and I had some very elementary training in management while there. I’ve had quite a lot of prior industry experience, which put me in a better place to work out what ‘good’ looks like, it helps me with fundraising strategies, with who we speak to, and how we pitch it, and what we think industry is looking for.
At Etcembly, myself and my co-founder have a pretty clear idea what that looks like. We’re also very fortunate to be guided by the founders of our predecessor company Immunocore who are our mentors, sit on our board and are our investors as well. It’s a privileged position to be in, but it’s also the ultimate endorsement if your former boss says he’ll invest in your company.
As a mother of three you have to be ruthless with your time. It has taught me to be extremely compartmentalized with my tasks. I had to teach myself how to multitask but also compartmentalize each task such that they don’t interfere with my personal life. Between Etcembly and Synaptix there’s a healthy firewall. It’s not easy to do. But if you put your mind to it, it can be done.
Q: What are you trying to achieve at Etcembly?
MT: With Etcembly, when we first started, we went straight into lockdown. That’s when we really did look at the entire TCR landscape. Other than Immunocore, no one can make picomolar TCR. They use a technology called phage display. Phage display has been around for decades and was pioneered by my old boss, John McCafferty. It has evolved to a certain extent, but there are limitations.
Immunocore has stayed with phage display, which works, but we want to look at what ‘better’ looks like. The current process is slow and laborious. It traditionally takes two years from discovery to getting your final product. Using machine learning, we’ve invented a new technology where we can discover and engineer a TCR in 11 months. We are building the ship as we are sailing it, so in that respect we have every reason to believe that when we do it the second and third time it will be even faster than that.
It’s about reinventing how we do things rather than just being contented with how something has always worked in the past. You have to evolve the technology and you need to find novel ways of solving a problem.
Q: Are TCR-based therapeutics going to be the new CAR T-cell therapies?
MT: I think I think that CAR T-cell therapy is a tried and tested technology. They have been very successful in treating liquid tumors, but they haven’t really seen a success with solid tumors, which is something that needs to be addressed. I think TCR therapy is the way forward for treating solid tumors that have high unmet need.
If you look at Immunocore’s first TCR product on the market, Kimmtrak, against uveal melanoma, it was already seen as a rare cancer indication, but their sales figures have actually exceeded expectations. It would suggest that the patient numbers are actually much higher than one would expect. On the back of Kimmtrak, Immunocore has another program targeting PRAME, which is the TCR we’ve engineered against.
PRAME is a $10 billion market, it is huge. Just in the US alone, there are at least 100,000 patients that would qualify for this treatment. Immunocore is obviously first to market. They are the front runner here, because they have product being tested in the clinics right now, that is doing very well. But we are a fast follower. First to the market is good, but there’s nothing to say that fast followers don’t do just as well, if not even better. If you take the analogy of Apple, they are not the first people to invent the iPad, or music streaming services, for example, but they’re doing extremely well, they are one of the market leaders.
Q: How are you using AI to help discover and develop these new therapies?
MT: One of the key methods that we use is large language models. Essentially, it’s training a machine using tokenized words. So, if you say, the fox jumped over the fence, if you blank out the last word, it could be fence, it could be moon, it can be anything. It’s analogous in the case of amino acids in the TCR sequence. You mark every single position of the amino acids, then the machine can learn what amino acid should occupy that position. It will come up with its own TCR sequence based on what’s available in our database.
It’s a bit like ChatGPT when you tell the machine to find something for you, and it gives you answers, but the answers can be slightly different every single time because there is a creative element to it. That’s the beauty of it. You get multiple options and you have a panel of potential candidates that we can then validate in the lab. We don’t take the predictions as ground truth; we always test them empirically to back up what is predicted by the machine.
We are one of the early adopters using machine learning to understand TCR and using it to engineer TCRs for immunotherapy. I don’t think anyone’s really applied it in that way before. I think it’s opened up a whole new field, it’s very interdisciplinary you need biologists and machine learning engineers, bioinformaticians and protein engineers to all talk to each other and find a common solution.
Q: What is next for Etcembly?
MT: It’s a really exciting time for the company. Right now, we are wrapping up fundraising, which has gone really well. But we also are looking to develop the pipeline. We’ll be taking our machine learning engineered TCR to the clinic and will be carrying out the preclinical development stage of that program. We will probably be expanding the pipeline to include other cancer targets and we’ll also be looking at the autoimmune space in the coming year or two. It feels like we’ve been a baby for a while and we’re suddenly growing up because we’ve been successful at what we have been trying to do.
This is just the tip of the iceberg. What we’ve just uncovered, what we can do. I think this will open up a whole new field and whole new era of emerging targets. We are also looking in the blood of long-term cancer survivors for new therapeutic modalities. I think lots of companies have not even thought about that. People who have been successfully treated with immunotherapy clearly have TCRs in the blood that can keep the cancer at bay This is also an area that we want to launch into.
Q: What advice would you give someone who wanted to launch a biotech company?
MT: I think you need to do your homework. Don’t just take the first offer that lands on your table. Assess the landscape and the market conditions. Who you bring on as an investor can make or break your company. We’ve been very fortunate that our investor base is extremely supportive, but we’ve also heard stories where companies have not been that fortunate. Whether it’s an institutional or angel investor you must always understand where they’re coming from, and what they want out of the investment. It’s great to have a wonderful idea that people will fund, but you must be very clear what your exit strategy is with yourself and with your investors because they need to be on the same page as you about what a good investment looks like.
Q: There’s been a lot of talk about needing more women in biotech and pharma, particularly at senior levels. What’s your position on the topic?
MT: I think the female biotech CEO is a rare breed. I don’t see many of them. But, in recent times, I’m starting to see more and more women coming forward in leadership roles, I think we’re still so far away from where we should be in terms of equality and having a 50:50 split in the boardroom. But, I’ve always championed and supported women in leadership positions. It’s my desire to see more women moving up the ranks in leadership roles and having a say in the boardroom. We’ve come very far, but there’s still a lot of work to do. I think the tides are slowly turning, but we have to be patient. I would also say that women absolutely have to support each other, otherwise, there is no hope that we will ever achieve equal rights.
Q: What advice would you give yourself if you could go back in time to when you started in your career?
MT: I would say everything happens in its time and just be patient. It took me a long time to figure out what I really wanted to do. I would tell myself not to be afraid of who I really am and that growing up and growing older is about becoming who you are meant to be in the first place.
Helen Albert is senior editor at Inside Precision Medicine and a freelance science journalist. Prior to going freelance, she was editor-in-chief at Labiotech, an English-language, digital publication based in Berlin focusing on the European biotech industry. Before moving to Germany, she worked at a range of different science and health-focused publications in London. She was editor of The Biochemist magazine and blog, but also worked as a senior reporter at Springer Nature’s medwireNews for a number of years, as well as freelancing for various international publications. She has written for New Scientist, Chemistry World, Biodesigned, The BMJ, Forbes, Science Business, Cosmos magazine, and GEN. Helen has academic degrees in genetics and anthropology, and also spent some time early in her career working at the Sanger Institute in Cambridge before deciding to move into journalism.
The post Building T Cell Receptor Therapies with Generative AI appeared first on Inside Precision Medicine.
treatment fda preclinical therapy european germanyInternational
Net Zero, The Digital Panopticon, & The Future Of Food
Net Zero, The Digital Panopticon, & The Future Of Food
Authored by Colin Todhunter via Off-Guardian.org,
The food transition, the energy…

Authored by Colin Todhunter via Off-Guardian.org,
The food transition, the energy transition, net-zero ideology, programmable central bank digital currencies, the censorship of free speech and clampdowns on protest. What’s it all about? To understand these processes, we need to first locate what is essentially a social and economic reset within the context of a collapsing financial system.
Writer Ted Reece notes that the general rate of profit has trended downwards from an estimated 43% in the 1870s to 17% in the 2000s. By late 2019, many companies could not generate enough profit. Falling turnover, squeezed margins, limited cashflows and highly leveraged balance sheets were prevalent.
Professor Fabio Vighi of Cardiff University has described how closing down the global economy in early 2020 under the guise of fighting a supposedly new and novel pathogen allowed the US Federal Reserve to flood collapsing financial markets (COVID relief) with freshly printed money without causing hyperinflation. Lockdowns curtailed economic activity, thereby removing demand for the newly printed money (credit) in the physical economy and preventing ‘contagion’.
According to investigative journalist Michael Byrant, €1.5 trillion was needed to deal with the crisis in Europe alone. The financial collapse staring European central bankers in the face came to a head in 2019. The appearance of a ‘novel virus’ provided a convenient cover story.
The European Central Bank agreed to a €1.31 trillion bailout of banks followed by the EU agreeing to a €750 billion recovery fund for European states and corporations. This package of long-term, ultra-cheap credit to hundreds of banks was sold to the public as a necessary programme to cushion the impact of the pandemic on businesses and workers.
In response to a collapsing neoliberalism, we are now seeing the rollout of an authoritarian great reset — an agenda that intends to reshape the economy and change how we live.
SHIFT TO AUTHORITARIANISM
The new economy is to be dominated by a handful of tech giants, global conglomerates and e-commerce platforms, and new markets will also be created through the financialisation of nature, which is to be colonised, commodified and traded under the notion of protecting the environment.
In recent years, we have witnessed an overaccumulation of capital, and the creation of such markets will provide fresh investment opportunities (including dodgy carbon offsetting Ponzi schemes) for the super-rich to park their wealth and prosper.
This great reset envisages a transformation of Western societies, resulting in permanent restrictions on fundamental liberties and mass surveillance. Being rolled out under the benign term of a ‘Fourth Industrial Revolution’, the World Economic Forum (WEF) says the public will eventually ‘rent’ everything they require (remember the WEF video ‘you will own nothing and be happy’?): stripping the right of ownership under the guise of a ‘green economy’ and underpinned by the rhetoric of ‘sustainable consumption’ and ‘climate emergency’.
Climate alarmism and the mantra of sustainability are about promoting money-making schemes. But they also serve another purpose: social control.
Neoliberalism has run its course, resulting in the impoverishment of large sections of the population. But to dampen dissent and lower expectations, the levels of personal freedom we have been used to will not be tolerated. This means that the wider population will be subjected to the discipline of an emerging surveillance state.
To push back against any dissent, ordinary people are being told that they must sacrifice personal liberty in order to protect public health, societal security (those terrible Russians, Islamic extremists or that Sunak-designated bogeyman George Galloway) or the climate. Unlike in the old normal of neoliberalism, an ideological shift is occurring whereby personal freedoms are increasingly depicted as being dangerous because they run counter to the collective good.
The real reason for this ideological shift is to ensure that the masses get used to lower living standards and accept them. Consider, for instance, the Bank of England’s chief economist Huw Pill saying that people should ‘accept’ being poorer. And then there is Rob Kapito of the world’s biggest asset management firm BlackRock, who says that a “very entitled” generation must deal with scarcity for the first time in their lives.
At the same time, to muddy the waters, the message is that lower living standards are the result of the conflict in Ukraine and supply shocks that both the war and ‘the virus’ have caused.
The net-zero carbon emissions agenda will help legitimise lower living standards (reducing your carbon footprint) while reinforcing the notion that our rights must be sacrificed for the greater good. You will own nothing, not because the rich and their neoliberal agenda made you poor but because you will be instructed to stop being irresponsible and must act to protect the planet.
NET-ZERO AGENDA
But what of this shift towards net-zero greenhouse gas emissions and the plan to slash our carbon footprints? Is it even feasible or necessary?
Gordon Hughes, a former World Bank economist and current professor of economics at the University of Edinburgh, says in a new report that current UK and European net-zero policies will likely lead to further economic ruin.
Apparently, the only viable way to raise the cash for sufficient new capital expenditure (on wind and solar infrastructure) would be a two decades-long reduction in private consumption of up to 10 per cent. Such a shock has never occurred in the last century outside war; even then, never for more than a decade.
But this agenda will also cause serious environmental degradation. So says Andrew Nikiforuk in the article The Rising Chorus of Renewable Energy Skeptics, which outlines how the green techno-dream is vastly destructive.
He lists the devastating environmental impacts of an even more mineral-intensive system based on renewables and warns:
“The whole process of replacing a declining system with a more complex mining-based enterprise is now supposed to take place with a fragile banking system, dysfunctional democracies, broken supply chains, critical mineral shortages and hostile geopolitics.”
All of this assumes that global warming is real and anthropogenic. Not everyone agrees. In the article Global warming and the confrontation between the West and the rest of the world, journalist Thierry Meyssan argues that net zero is based on political ideology rather than science. But to state such things has become heresy in the Western countries and shouted down with accusations of ‘climate science denial’.
Regardless of such concerns, the march towards net zero continues, and key to this is the United Nations Agenda 2030 for Sustainable Development Goals.
Today, almost every business or corporate report, website or brochure includes a multitude of references to ‘carbon footprints’, ‘sustainability’, ‘net zero’ or ‘climate neutrality’ and how a company or organisation intends to achieve its sustainability targets. Green profiling, green bonds and green investments go hand in hand with displaying ‘green’ credentials and ambitions wherever and whenever possible.
It seems anyone and everyone in business is planting their corporate flag on the summit of sustainability. Take Sainsbury’s, for instance. It is one of the ‘big six’ food retail supermarkets in the UK and has a vision for the future of food that it published in 2019.
Here’s a quote from it:
“Personalised Optimisation is a trend that could see people chipped and connected like never before. A significant step on from wearable tech used today, the advent of personal microchips and neural laces has the potential to see all of our genetic, health and situational data recorded, stored and analysed by algorithms which could work out exactly what we need to support us at a particular time in our life. Retailers, such as Sainsbury’s could play a critical role to support this, arranging delivery of the needed food within thirty minutes — perhaps by drone.”
Tracked, traced and chipped — for your own benefit. Corporations accessing all of our personal data, right down to our DNA. The report is littered with references to sustainability and the climate or environment, and it is difficult not to get the impression that it is written so as to leave the reader awestruck by the technological possibilities.
However, the promotion of a brave new world of technological innovation that has nothing to say about power — who determines policies that have led to massive inequalities, poverty, malnutrition, food insecurity and hunger and who is responsible for the degradation of the environment in the first place — is nothing new.
The essence of power is conveniently glossed over, not least because those behind the prevailing food regime are also shaping the techno-utopian fairytale where everyone lives happily ever after eating bugs and synthetic food while living in a digital panopticon.
FAKE GREEN
The type of ‘green’ agenda being pushed is a multi-trillion market opportunity for lining the pockets of rich investors and subsidy-sucking green infrastructure firms and also part of a strategy required to secure compliance required for the ‘new normal’.
It is, furthermore, a type of green that plans to cover much of the countryside with wind farms and solar panels with most farmers no longer farming. A recipe for food insecurity.
Those investing in the ‘green’ agenda care first and foremost about profit. The supremely influential BlackRock invests in the current food system that is responsible for polluted waterways, degraded soils, the displacement of smallholder farmers, a spiralling public health crisis, malnutrition and much more.
It also invests in healthcare — an industry that thrives on the illnesses and conditions created by eating the substandard food that the current system produces. Did Larry Fink, the top man at BlackRock, suddenly develop a conscience and become an environmentalist who cares about the planet and ordinary people? Of course not.
Any serious deliberations on the future of food would surely consider issues like food sovereignty, the role of agroecology and the strengthening of family farms — the backbone of current global food production.
The aforementioned article by Andrew Nikiforuk concludes that, if we are really serious about our impacts on the environment, we must scale back our needs and simplify society.
In terms of food, the solution rests on a low-input approach that strengthens rural communities and local markets and prioritises smallholder farms and small independent enterprises and retailers, localised democratic food systems and a concept of food sovereignty based on self-sufficiency, agroecological principles and regenerative agriculture.
It would involve facilitating the right to culturally appropriate food that is nutritionally dense due to diverse cropping patterns and free from toxic chemicals while ensuring local ownership and stewardship of common resources like land, water, soil and seeds.
That’s where genuine environmentalism and the future of food begins.
Government
Five Aerospace Investments to Buy as Wars Worsen Copy
Five aerospace investments to buy as wars worsen give investors a chance to acquire shares of companies focused on fortifying national defense. The five…
Five aerospace investments to buy as wars worsen give investors a chance to acquire shares of companies focused on fortifying national defense.
The five aerospace investments to buy provide military products to help protect freedom amid Russia’s ongoing onslaught against Ukraine that began in February 2022, as well as supply arms in the Middle East used after Hamas militants attacked and murdered civilians in Israel on Oct. 7. Even though the S&P 500 recently reached all-time highs, these five aerospace investments have remained reasonably priced and rated as recommendations by seasoned analysts and a pension fund chairman.
State television broadcasts in Russia show the country’s soldiers advancing further into Ukrainian territory, but protests have occurred involving family members of those serving in perilous conditions in the invasion of their neighboring nation to be brought home. Even though hundreds of thousands of Russians also have fled to other countries to avoid compulsory military service, the aggressor’s President Vladimir Putin has vowed to continue to send additional soldiers into the fierce fighting.
While Russia’s land-grab of Crimea and other parts of Ukraine show no end in sight, Israel’s war with Hamas likely will last for at least additional months, according to the latest reports. United Nations’ leaders expressed alarm on Dec. 26 about intensifying Israeli attacks that killed more than 100 Palestinians over two days in part of the Gaza Strip, when 15 members of the Israel Defense Force (IDF) also lost their lives.
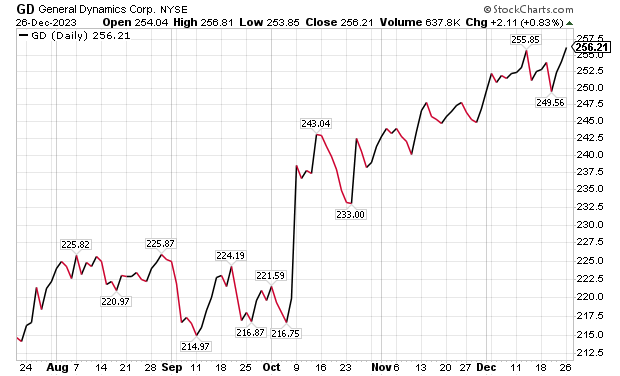
Five Aerospace Investments to Buy as Wars Worsen: General Dynamics
One of the five aerospace investments to buy as wars worsen is General Dynamics (NYSE: GD), a Reston, Virginia-based aerospace company with more than 100,000 employees in 70-plus countries. A key business unit of General Dynamics is Gulfstream Aerospace Corporation, a manufacturer of business aircraft. Other segments of General Dynamics focus on making military products such as Abrams tanks, Stryker fighting vehicles, ASCOD fighting vehicles like the Spanish PIZARRO and British AJAX, LAV-25 Light Armored Vehicles and Flyer-60 lightweight tactical vehicles.
For the U.S. Navy and other allied armed forces, General Dynamics builds Virginia-class attack submarines, Columbia-class ballistic missile submarines, Arleigh Burke-class guided missile destroyers, Expeditionary Sea Base ships, fleet logistics ships, commercial cargo ships, aircraft and naval gun systems, Hydra-70 rockets, military radios and command and control systems. In addition, the company provides radio and optical telescopes, secure mobile phones, PIRANHA and PANDUR wheeled armored vehicles and mobile bridge systems.
Chicago-based investment firm William Blair & Co. is among those recommending General Dynamics. The Chicago firm gave an “outperform” rating to General Dynamics in a Dec. 21 research note.
Gulfstream is seeking G700 FAA certification by the end of 2023, suggesting potentially positive news in the next 10 days, William Blair wrote in its recent research note. The investment firm projected that General Dynamics would trade upward upward upon the G700’s certification.
“General Dynamics’ 2023 aircraft delivery guidance of approximately 134 planes assumes that 19 G700s are delivered in the fourth quarter,” wrote William Blair’s aerospace and defense analyst Louie DiPalma. “Even if deliveries fall short of this target, we believe investors will take a glass-half-full approach upon receipt of the certification.”
Chart courtesy of www.stockcharts.com.
Five Aerospace Investments to Buy as Wars Worsen: GD Outlook
The G700 is a major focus area for investors because it is Gulfstream’s most significant aircraft introduction since the iconic G650 in 2012, DiPalma wrote. Gulfstream has the highest market share in the long-range jet segment of the private aircraft market, the highest profit margin of aircraft peers and the most premium business aviation brand, he added.
“The aircraft remains immensely popular today with corporations and high-net-worth individuals,” Di Palma wrote. “Elon Musk has reportedly placed an order for a G700 to go along with his existing G650. Qatar Airways announced at the Paris Air Show that 10 G700 aircraft will become part of its fleet.”
G700 deliveries and subsequent G800 deliveries are expected to be the cornerstone of Gulfstream’s growth and margin expansion for the next decade, DiPalma wrote. This should lead to a rebound in the stock price as the margins for the G700 and G800 are very attractive, he added.
Management’s guidance is for the aerospace operating margin to increase from about 13.2% in 2022 to roughly 14.0% in 2023 and 15.8% in 2024. Longer term, a high-teens profit margin appears within reach, DiPalma projected.
In other General Dynamics business segments, William Blair expects several yet-unannounced large contract awards for General Dynamics IT, to go along with C$1.7 billion, or US$1.29 billion, in General Dynamics Mission Systems contracts announced on Dec. 20 for the Canadian Army. General Dynamics shares are poised to have a strong 2024, William Blair wrote.
Five Aerospace Investments to Buy as Wars Worsen: VSE Corporation
Alexandria, Virginia-based VSE Corporation’s (NASDAQ: VSEC) price-to-earnings (P/E) valuation multiple of 22 received support when AAR Corp. (NYSE: AIR), a Wood Dale, Illinois, provider of aviation services, announced on Dec. 21 that it would acquire the product support business of Triumph Group (NYSE: TGI), a Berwyn, Pennsylvania, supplier of aerospace services, structures and systems. AAR’s purchase price of $725 million reflects confidence in a continued post-pandemic aerospace rebound.
VSE, a provider of aftermarket distribution and repair services for land, sea and air transportation assets used by government and commercial markets, is rated “outperform” by William Blair. The company’s core services include maintenance, repair and operations (MRO), parts distribution, supply chain management and logistics, engineering support, as well as consulting and training for global commercial, federal, military and defense customers.
“Robust consumer travel demand and aging aircraft fleets have driven elevated maintenance visits,” William Blair’s DiPalma wrote in a Dec. 21 research note. “The AAR–Triumph deal is valued at a premium 13-times 2024 EBITDA multiple, which was in line with the valuation multiple that Heico (NYSE: HEI) paid for Wencor over the summer.”
VSE currently trades at a discounted 9.5 times consensus 2024 earnings before interest, taxes, depreciation and amortization (EBITDA) estimates, as well as 11.6 times consensus 2023 EBITDA.
Five Aerospace Investments to Buy as Wars Worsen: VSE Undervalued?
“We expect that VSE shares will trend higher as investors process this deal,” DiPalma wrote. “VSE shares trade at 9.5 times consensus 2024 adjusted EBITDA, compared with peers and M&A comps in the 10-to-14-times range. We think that VSE’s multiple will expand as it closes the divestiture of its federal and defense business and makes strategic acquisitions. We see consistent 15% annual upside for shares as VSE continues to take share in the $110 billion aviation aftermarket industry.”
William Blair reaffirmed its “outperform” rating for VSE on Dec. 21. The main risk to VSE shares is lumpiness associated with its aviation services margins, Di Palma wrote. However, he raised 2024 estimates to further reflect commentary from VSE’s analysts’ day in November.

Chart courtesy of www.stockcharts.com.
Five Aerospace Investments to Buy as Wars Worsen: HEICO Corporation
HEICO Corporation (NYSEL: HEI), is a Hollywood, Florida-based technology-driven aerospace, industrial, defense and electronics company that also is ranked as an “outperform” investment by William Blair’s DiPalma. The aerospace aftermarket parts provider recently reported fourth-quarter financials above consensus analysts’ estimates, driven by 20% organic growth in HEICO’s flight support group.
HEICO’s management indicated that the performance of recently acquired Wencor is exceeding expectations. However, HEICO leaders offered color on 2024 organic growth and margin expectations that forecast reduced gains. Even though consensus estimates already assumed slowing growth, it is still not a positive for HEICO, DiPalma wrote.
William Blair forecasts 15% annual upside to HEICO’s shares, based on EBITDA growth. HEICO’s management cited a host of reasons for its quarterly outperformance, highlighted by the continued commercial air travel recovery. The company also referenced new product introductions and efficiency initiatives.
HEICO’s defense product sales increased by 26% sequentially, marking the third consecutive sequential increase in defense product revenue. The company’s leaders conveyed that defense in general is moving in the right direction to enhance financial performance.

Chart courtesy of www.stockcharts.com.
Five Dividend-paying Defense and Aerospace Investments to Purchase: XAR
A fourth way to obtain exposure to defense and aerospace investments is through SPDR S&P Aerospace and Defense ETF (XAR). That exchange-traded fund tracks the S&P Aerospace & Defense Select Industry Index. The fund is overweight in industrials and underweight in technology and consumer cyclicals, said Bob Carlson, a pension fund chairman who heads the Retirement Watch investment newsletter.

Bob Carlson, who heads Retirement Watch, answers questions from Paul Dykewicz.
XAR has 34 securities, and 44.2% of the fund is in the 10 largest positions. The fund is up 25.82% in the last 12 months, 22.03% in the past three months and 7.92% for the last month. Its dividend yield recently measured 0.38%.
The largest positions in the fund recently were Axon Enterprise (NASDAQ: AXON), Boeing (NYSE: BA), L3Harris Technologies (NYSE: LHX), Spirit Aerosystems (NYSE: SPR) and Virgin Galactic (NYSE: SPCE).

Chart courtesy of www.stockcharts.com
Five Dividend-paying Defense and Aerospace Investments to Purchase: PPA
The second fund recommended by Carlson is Invesco Aerospace & Defense ETF (PPA), which tracks the SPADE Defense Index. It has the same underweighting and overweighting as XAR, he said.
PPA recently held 52 securities and 53.2% of the fund was in its 10 largest positions. With so many holdings, the fund offers much reduced risk compared to buying individual stocks. The largest positions in the fund recently were Boeing (NYSE: BA), RTX Corp. (NYSE: RTX), Lockheed Martin (NYSE: LMT), Northrop Grumman (NYSE: NOC) and General Electric (NYSE:GE).
The fund is up 19.07% for the past year, 50.34% in the last three months and 5.30% during the past month. The dividend yield recently touched 0.69%.

Chart courtesy of www.stockcharts.com
Other Fans of Aerospace
Two fans of aerospace stocks are Mark Skousen, PhD, and seasoned stock picker Jim Woods. The pair team up to head the Fast Money Alert advisory service They already are profitable in their recent recommendation of Lockheed Martin (NYSE: LMT) in Fast Money Alert.

Mark Skousen, a scion of Ben Franklin, meets with Paul Dykewicz.

Jim Woods, a former U.S. Army paratrooper, co-heads Fast Money Alert.
Bryan Perry, who heads the Cash Machine investment newsletter and the Micro-Cap Stock Trader advisory service, recommends satellite services provider Globalstar (NYSE American: GSAT), of Covington, Louisiana, that has jumped 50.00% since he advised buying it two months ago. Perry is averaging a dividend yield of 11.14% in his Cash Machine newsletter but is breaking out with the red-hot recommendation of Globalstar in his Micro-Cap Stock Trader advisory service.

Bryan Perry heads Cash Machine, averaging an 11.14% dividend yield.
Military Equipment Demand Soars amid Multiple Wars
The U.S. military faces an acute need to adopt innovation, to expedite implementation of technological gains, to tap into the talents of people in various industries and to step-up collaboration with private industry and international partners to enhance effectiveness, U.S. Joint Chiefs of Staff Gen. Charles Q. Brown Jr. told attendees on Nov 16 at a national security conference. Prime examples of the need are showed by multiple raging wars, including the Middle East and Ukraine. A cold war involves China and its increasingly strained relationships with Taiwan and other Asian nations.
The shocking Oct. 7 attack by Hamas on Israel touched off an ongoing war in the Middle East, coupled with Russia’s February 2022 invasion and continuing assault of neighboring Ukraine. Those brutal military conflicts show the fragility of peace when determined aggressors are willing to use any means necessary to achieve their goals. To fend off such attacks, rapid and effective response is required.
“The Department of Defense is doing more than ever before to deter, defend, and, if necessary, defeat aggression,” Gen. Brown said at the National Security Innovation Forum at the Johns Hopkins University Bloomberg Center in Washington, D.C.
One of Russia’s war ships, the 360-foot-long Novocherkassk, was damaged on Dec. 26 by a Ukrainian attack on the Black Sea port of Feodosia in Crimea. This video of an explosion at the port that reportedly shows a section of the ship hit by aircraft-guided missiles.

Chairman Joint Chiefs of Staff Gen. Charles Q. Brown, Jr.
Photo By: Benjamin Applebaum
National security threats can compel immediate action, Gen. Brown said he quickly learned since taking his post on Oct. 1.
“We may not have much warning when the next fight begins,” Gen. Brown said. “We need to be ready.”
In a pre-recorded speech at the national security conference, Michael R. Bloomberg, founder of Bloomberg LP, told the John Hopkins national security conference attendees about the critical need for collaboration between government and industry.
“Building enduring technological advances for the U.S. military will help our service members and allies defend freedom across the globe,” Bloomberg said.
The “horrific terrorist attacks” against Israel and civilians living there on Oct. 7 underscore the importance of that mission, Bloomberg added.
Paul Dykewicz, www.pauldykewicz.com, is an accomplished, award-winning journalist who has written for Dow Jones, the Wall Street Journal, Investor’s Business Daily, USA Today, the Journal of Commerce, Seeking Alpha, Guru Focus and other publications and websites. Attention Holiday Gift Buyers! Consider purchasing Paul’s inspirational book, “Holy Smokes! Golden Guidance from Notre Dame’s Championship Chaplain,” with a foreword by former national championship-winning football coach Lou Holtz. The uplifting book is great gift and is endorsed by Joe Montana, Joe Theismann, Ara Parseghian, “Rocket” Ismail, Reggie Brooks, Dick Vitale and many others. Call 202-677-4457 for special pricing on multiple-book purchases or autographed copies! Follow Paul on Twitter @PaulDykewicz. He is the editor of StockInvestor.com and DividendInvestor.com, a writer for both websites and a columnist. He further is editorial director of Eagle Financial Publications in Washington, D.C., where he edits monthly investment newsletters, time-sensitive trading alerts, free e-letters and other investment reports. Paul previously served as business editor of Baltimore’s Daily Record newspaper, after writing for the Baltimore Business Journal and Crain Communications.
The post Five Aerospace Investments to Buy as Wars Worsen Copy appeared first on Stock Investor.
dow jones sp 500 nasdaq stocks pandemic etf micro-cap army recovery russia ukraine chinaGovernment
Health Officials: Man Dies From Bubonic Plague In New Mexico
Health Officials: Man Dies From Bubonic Plague In New Mexico
Authored by Jack Phillips via The Epoch Times (emphasis ours),
Officials in…
Authored by Jack Phillips via The Epoch Times (emphasis ours),
Officials in New Mexico confirmed that a resident died from the plague in the United States’ first fatal case in several years.
The New Mexico Department of Health, in a statement, said that a man in Lincoln County “succumbed to the plague.” The man, who was not identified, was hospitalized before his death, officials said.
They further noted that it is the first human case of plague in New Mexico since 2021 and also the first death since 2020, according to the statement. No other details were provided, including how the disease spread to the man.
The agency is now doing outreach in Lincoln County, while “an environmental assessment will also be conducted in the community to look for ongoing risk,” the statement continued.
“This tragic incident serves as a clear reminder of the threat posed by this ancient disease and emphasizes the need for heightened community awareness and proactive measures to prevent its spread,” the agency said.
A bacterial disease that spreads via rodents, it is generally spread to people through the bites of infected fleas. The plague, known as the black death or the bubonic plague, can spread by contact with infected animals such as rodents, pets, or wildlife.
The New Mexico Health Department statement said that pets such as dogs and cats that roam and hunt can bring infected fleas back into homes and put residents at risk.
Officials warned people in the area to “avoid sick or dead rodents and rabbits, and their nests and burrows” and to “prevent pets from roaming and hunting.”
“Talk to your veterinarian about using an appropriate flea control product on your pets as not all products are safe for cats, dogs or your children” and “have sick pets examined promptly by a veterinarian,” it added.
“See your doctor about any unexplained illness involving a sudden and severe fever, the statement continued, adding that locals should clean areas around their home that could house rodents like wood piles, junk piles, old vehicles, and brush piles.
The plague, which is spread by the bacteria Yersinia pestis, famously caused the deaths of an estimated hundreds of millions of Europeans in the 14th and 15th centuries following the Mongol invasions. In that pandemic, the bacteria spread via fleas on black rats, which historians say was not known by the people at the time.
Other outbreaks of the plague, such as the Plague of Justinian in the 6th century, are also believed to have killed about one-fifth of the population of the Byzantine Empire, according to historical records and accounts. In 2013, researchers said the Justinian plague was also caused by the Yersinia pestis bacteria.
But in the United States, it is considered a rare disease and usually occurs only in several countries worldwide. Generally, according to the Mayo Clinic, the bacteria affects only a few people in U.S. rural areas in Western states.
Recent cases have occurred mainly in Africa, Asia, and Latin America. Countries with frequent plague cases include Madagascar, the Democratic Republic of Congo, and Peru, the clinic says. There were multiple cases of plague reported in Inner Mongolia, China, in recent years, too.
Symptoms
Symptoms of a bubonic plague infection include headache, chills, fever, and weakness. Health officials say it can usually cause a painful swelling of lymph nodes in the groin, armpit, or neck areas. The swelling usually occurs within about two to eight days.
The disease can generally be treated with antibiotics, but it is usually deadly when not treated, the Mayo Clinic website says.
“Plague is considered a potential bioweapon. The U.S. government has plans and treatments in place if the disease is used as a weapon,” the website also says.
According to data from the U.S. Centers for Disease Control and Prevention, the last time that plague deaths were reported in the United States was in 2020 when two people died.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International6 days ago
International6 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International6 days ago
International6 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















