Braxia Scientific Launches KetaMD in Florida; First Patients Complete Initial Virtual Ketamine Treatments At-Home
Braxia Scientific Launches KetaMD in Florida; First Patients Complete Initial Virtual Ketamine Treatments At-Home
Canada NewsWire
TORONTO, Sept. 12, 2022
TORONTO, Sept. 12, 2022 /CNW Telbec/ – Braxia Scientific Corp. (“Braxia Scientific”, or the “C…

Braxia Scientific Launches KetaMD in Florida; First Patients Complete Initial Virtual Ketamine Treatments At-Home
Canada NewsWire
TORONTO, Sept. 12, 2022
TORONTO, Sept. 12, 2022 /CNW Telbec/ - Braxia Scientific Corp. ("Braxia Scientific", or the "Company") (CSE: BRAX) (OTC: BRAXF) (FWB: 4960), a medical research and telemedicine company with clinics providing innovative ketamine and psilocybin treatments for depression and related disorders, is pleased to announce it has successfully launched KetaMD in Florida. KetaMD onboarded new patients and completed initial ketamine treatments. Eligible patients, as determined by one of KetaMD's licensed Florida healthcare practitioners, received medically supervised treatments, guided virtually by registered nurses with mental health expertise, while in the comfort of their home.
KetaMD, Inc. ("KetaMD"), a wholly owned subsidiary of Braxia Scientific, is an innovative end-to-end telemedicine platform, that provides access to safe, affordable, reliable and potentially life-changing at-home ketamine treatments for people suffering from mental health disorders such as depression, bipolar disorder, anxiety and post-traumatic stress disorder (PTSD).
In multiple studies, ketamine has also been shown to have rapid and persistent benefits for acute care in suicidal patients who often suffer from mental health challenges. As National Suicide Awareness month kicks off, KetaMD has ramped up operations and session availability to provide additional support to Florida residents in need of treatment.
Dr. Roger McIntyre commented, "We are incredibly proud to have treated our first patients in the U.S. through our KetaMD platform. KetaMD solves considerable issues by increasing access to innovative ketamine treatments for patients suffering from mental health disorders who don't live close to a care provider, have difficulty accessing care, or are unable to go to the doctor because of circumstances like COVID-19."
Warren Gumpel, CEO of KetaMD commented, "We started building the model for KetaMD more than five years ago with the goal of deploying the latest technology to improve people's lives and to empower those living with depression and other mental health conditions to take steps necessary to seek and receive safe, high quality, affordable, reliable care. We are working on scaling KetaMD beyond Florida across the U.S. opening up the opportunity for people to receive care anytime, from the comfort of their own homes."
For those interested in qualifying for KetaMD are encouraged to visit www.KetaMD.com.
KetaMD is a HIPAA-compliant telemedicine platform providing affordable and potentially life-changing at-home medical ketamine treatments. Prescribed by medical professionals and guided by registered nurses via our secure platform, KetaMD is a safe and effective alternative for the millions of adults that currently rely on conventional mental health treatments to relieve depression, anxiety, and related mental health challenges. Our mission is to make this treatment safely accessible to those who need it across the United States. Through KetaMD's program, adults who receive a qualifying diagnosis from one of our licensed medical prescribers may participate in our fully virtual program. For more information, visit www.ketamd.com.
Braxia Scientific is a medical research and telemedicine company with clinics that provide innovative ketamine and psilocybin treatments for people with depression and related disorders. Braxia also launched its U.S. based end-to-end telemedicine platform KetaMD utilizing leading technology to provide access to safe, affordable, and potentially life-changing at-home ketamine treatments for people living with depression and related mental health conditions. Through its medical solutions, Braxia aims to reduce the illness burden of brain-based disorders, such as major depressive disorder among others. Braxia is primarily focused on (i) owning and operating multidisciplinary clinics, providing treatments in-person and virtually for mental health disorders, and (ii) research activities related to discovering and commercializing novel drugs and delivery methods. Braxia seeks to develop ketamine and derivatives and other psychedelic products from its IP development platform. Through its wholly owned subsidiary, Braxia Health (formerly the Canadian Rapid Treatment Center of Excellence Inc.), operates multidisciplinary community-based clinics offering rapid-acting treatments for depression located in Mississauga, Toronto, Kitchener-Waterloo, Ottawa, and Montreal.
ON BEHALF OF THE BOARD
"Dr. Roger S. McIntyre"
Dr. Roger S. McIntyre
Chairman & CEO
The CSE has not reviewed and does not accept responsibility for the accuracy or adequacy of this release.
This news release contains forward-looking statements within the meaning of applicable securities laws. All statements that are not historical facts, future estimates, plans, programs, forecasts, projections, objectives, assumptions, expectations, or beliefs of future performance are "forward-looking statements."
Forward-looking statements include statements about the intended promise of ketamine-based treatments for depression, the potential for ketamine or other psychedelics to treat other mental health conditions, the integration plans for Braxia and KetaMD, the intention to conduct further clinical trials, the expected growth of at-home telemedicine, the expected benefit and synergies of Braxia and KetaMD and the expectation to expand to areas other than Florida. Such forward- looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results, events, or developments to be materially different from any future results, events or developments expressed or implied by such forward-looking statements. Such risks and uncertainties include, among others, the failure of ketamine, psilocybin and other psychedelics to provide the expected health benefits and unanticipated side effects, dependence on obtaining and maintaining regulatory approvals, including acquiring and renewing federal, provincial, municipal, local or other licenses and engaging in activities that could be later determined to be illegal under domestic or international laws. Ketamine and psilocybin are currently Schedule I and Schedule III controlled substances, respectively, under the Controlled Drugs and Substances Act, S.C. 1996, c. 19 (the "CDSA") and it is a criminal offence to possess such substances under the CDSA without a prescription or a legal exemption. Health Canada has not approved psilocybin as a drug for any indication, however ketamine is a legally permissible medication for the treatment of certain psychological conditions. It is illegal to possess such substances in Canada without a prescription.
These factors should be considered carefully, and readers are cautioned not to place undue reliance on such forward-looking statements.
Although the Company has attempted to identify important risk factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other risk factors that cause actions, events or results to differ from those anticipated, estimated or intended. Additional information identifying risks and uncertainties that could affect financial results is contained in the Company's filings with Canadian securities regulators, including the Amended and Restated Listing Statement dated April 15, 2021 and its most recent MD&A, which are available at www.sedar.com. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in forward-looking statements.
Website: www.braxiascientific.com
View original content to download multimedia:https://www.prnewswire.com/news-releases/braxia-scientific-launches-ketamd-in-florida-first-patients-complete-initial-virtual-ketamine-treatments-at-home-301621751.html
SOURCE Braxia Scientific Corp.
Government
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
CDC Warns Thousands Of Children Sent To ER After Taking Common Sleep Aid
Authored by Jack Phillips via The Epoch Times (emphasis ours),
A…

Authored by Jack Phillips via The Epoch Times (emphasis ours),
A U.S. Centers for Disease Control (CDC) paper released Thursday found that thousands of young children have been taken to the emergency room over the past several years after taking the very common sleep-aid supplement melatonin.
The agency said that melatonin, which can come in gummies that are meant for adults, was implicated in about 7 percent of all emergency room visits for young children and infants “for unsupervised medication ingestions,” adding that many incidents were linked to the ingestion of gummy formulations that were flavored. Those incidents occurred between the years 2019 and 2022.
Melatonin is a hormone produced by the human body to regulate its sleep cycle. Supplements, which are sold in a number of different formulas, are generally taken before falling asleep and are popular among people suffering from insomnia, jet lag, chronic pain, or other problems.
The supplement isn’t regulated by the U.S. Food and Drug Administration and does not require child-resistant packaging. However, a number of supplement companies include caps or lids that are difficult for children to open.
The CDC report said that a significant number of melatonin-ingestion cases among young children were due to the children opening bottles that had not been properly closed or were within their reach. Thursday’s report, the agency said, “highlights the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight,” including melatonin.
The approximately 11,000 emergency department visits for unsupervised melatonin ingestions by infants and young children during 2019–2022 highlight the importance of educating parents and other caregivers about keeping all medications and supplements (including gummies) out of children’s reach and sight.
The CDC notes that melatonin use among Americans has increased five-fold over the past 25 years or so. That has coincided with a 530 percent increase in poison center calls for melatonin exposures to children between 2012 and 2021, it said, as well as a 420 percent increase in emergency visits for unsupervised melatonin ingestion by young children or infants between 2009 and 2020.
Some health officials advise that children under the age of 3 should avoid taking melatonin unless a doctor says otherwise. Side effects include drowsiness, headaches, agitation, dizziness, and bed wetting.
Other symptoms of too much melatonin include nausea, diarrhea, joint pain, anxiety, and irritability. The supplement can also impact blood pressure.
However, there is no established threshold for a melatonin overdose, officials have said. Most adult melatonin supplements contain a maximum of 10 milligrams of melatonin per serving, and some contain less.
Many people can tolerate even relatively large doses of melatonin without significant harm, officials say. But there is no antidote for an overdose. In cases of a child accidentally ingesting melatonin, doctors often ask a reliable adult to monitor them at home.
Dr. Cora Collette Breuner, with the Seattle Children’s Hospital at the University of Washington, told CNN that parents should speak with a doctor before giving their children the supplement.
“I also tell families, this is not something your child should take forever. Nobody knows what the long-term effects of taking this is on your child’s growth and development,” she told the outlet. “Taking away blue-light-emitting smartphones, tablets, laptops, and television at least two hours before bed will keep melatonin production humming along, as will reading or listening to bedtime stories in a softly lit room, taking a warm bath, or doing light stretches.”
In 2022, researchers found that in 2021, U.S. poison control centers received more than 52,000 calls about children consuming worrisome amounts of the dietary supplement. That’s a six-fold increase from about a decade earlier. Most such calls are about young children who accidentally got into bottles of melatonin, some of which come in the form of gummies for kids, the report said.
Dr. Karima Lelak, an emergency physician at Children’s Hospital of Michigan and the lead author of the study published in 2022 by the CDC, found that in about 83 percent of those calls, the children did not show any symptoms.
However, other children had vomiting, altered breathing, or other symptoms. Over the 10 years studied, more than 4,000 children were hospitalized, five were put on machines to help them breathe, and two children under the age of two died. Most of the hospitalized children were teenagers, and many of those ingestions were thought to be suicide attempts.
Those researchers also suggested that COVID-19 lockdowns and virtual learning forced more children to be at home all day, meaning there were more opportunities for kids to access melatonin. Also, those restrictions may have caused sleep-disrupting stress and anxiety, leading more families to consider melatonin, they suggested.
The Associated Press contributed to this report.
International
Red Candle In The Wind
Red Candle In The Wind
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by…
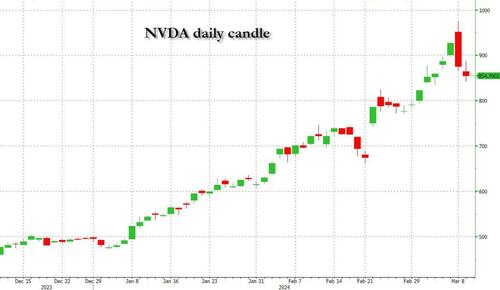
By Benjamin PIcton of Rabobank
February non-farm payrolls superficially exceeded market expectations on Friday by printing at 275,000 against a consensus call of 200,000. We say superficially, because the downward revisions to prior months totalled 167,000 for December and January, taking the total change in employed persons well below the implied forecast, and helping the unemployment rate to pop two-ticks to 3.9%. The U6 underemployment rate also rose from 7.2% to 7.3%, while average hourly earnings growth fell to 0.2% m-o-m and average weekly hours worked languished at 34.3, equalling pre-pandemic lows.
Undeterred by the devil in the detail, the algos sprang into action once exchanges opened. Market darling NVIDIA hit a new intraday high of $974 before (presumably) the humans took over and sold the stock down more than 10% to close at $875.28. If our suspicions are correct that it was the AIs buying before the humans started selling (no doubt triggering trailing stops on the way down), the irony is not lost on us.
The 1-day chart for NVIDIA now makes for interesting viewing, because the red candle posted on Friday presents quite a strong bearish engulfing signal. Volume traded on the day was almost double the 15-day simple moving average, and similar price action is observable on the 1-day charts for both Intel and AMD. Regular readers will be aware that we have expressed incredulity in the past about the durability the AI thematic melt-up, so it will be interesting to see whether Friday’s sell off is just a profit-taking blip, or a genuine trend reversal.
AI equities aside, this week ought to be important for markets because the BTFP program expires today. That means that the Fed will no longer be loaning cash to the banking system in exchange for collateral pledged at-par. The KBW Regional Banking index has so far taken this in its stride and is trading 30% above the lows established during the mini banking crisis of this time last year, but the Fed’s liquidity facility was effectively an exercise in can-kicking that makes regional banks a sector of the market worth paying attention to in the weeks ahead. Even here in Sydney, regulators are warning of external risks posed to the banking sector from scheduled refinancing of commercial real estate loans following sharp falls in valuations.
Markets are sending signals in other sectors, too. Gold closed at a new record-high of $2178/oz on Friday after trading above $2200/oz briefly. Gold has been going ballistic since the Friday before last, posting gains even on days where 2-year Treasury yields have risen. Gold bugs are buying as real yields fall from the October highs and inflation breakevens creep higher. This is particularly interesting as gold ETFs have been recording net outflows; suggesting that price gains aren’t being driven by a retail pile-in. Are gold buyers now betting on a stagflationary outcome where the Fed cuts without inflation being anchored at the 2% target? The price action around the US CPI release tomorrow ought to be illuminating.
Leaving the day-to-day movements to one side, we are also seeing further signs of structural change at the macro level. The UK budget last week included a provision for the creation of a British ISA. That is, an Individual Savings Account that provides tax breaks to savers who invest their money in the stock of British companies. This follows moves last year to encourage pension funds to head up the risk curve by allocating 5% of their capital to unlisted investments.
As a Hail Mary option for a government cruising toward an electoral drubbing it’s a curious choice, but it’s worth highlighting as cash-strapped governments increasingly see private savings pools as a funding solution for their spending priorities.
Of course, the UK is not alone in making creeping moves towards financial repression. In contrast to announcements today of increased trade liberalisation, Australian Treasurer Jim Chalmers has in the recent past flagged his interest in tapping private pension savings to fund state spending priorities, including defence, public housing and renewable energy projects. Both the UK and Australia appear intent on finding ways to open up the lungs of their economies, but government wants more say in directing private capital flows for state goals.
So, how far is the blurring of the lines between free markets and state planning likely to go? Given the immense and varied budgetary (and security) pressures that governments are facing, could we see a re-up of WWII-era Victory bonds, where private investors are encouraged to do their patriotic duty by directly financing government at negative real rates?
That would really light a fire under the gold market.
Government
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Fauci Deputy Warned Him Against Vaccine Mandates: Email
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19…

Authored by Zachary Stieber via The Epoch Times (emphasis ours),
Mandating COVID-19 vaccination was a mistake due to ethical and other concerns, a top government doctor warned Dr. Anthony Fauci after Dr. Fauci promoted mass vaccination.
“Coercing or forcing people to take a vaccine can have negative consequences from a biological, sociological, psychological, economical, and ethical standpoint and is not worth the cost even if the vaccine is 100% safe,” Dr. Matthew Memoli, director of the Laboratory of Infectious Diseases clinical studies unit at the U.S. National Institute of Allergy and Infectious Diseases (NIAID), told Dr. Fauci in an email.
“A more prudent approach that considers these issues would be to focus our efforts on those at high risk of severe disease and death, such as the elderly and obese, and do not push vaccination on the young and healthy any further.”
Employing that strategy would help prevent loss of public trust and political capital, Dr. Memoli said.
The email was sent on July 30, 2021, after Dr. Fauci, director of the NIAID, claimed that communities would be safer if more people received one of the COVID-19 vaccines and that mass vaccination would lead to the end of the COVID-19 pandemic.
“We’re on a really good track now to really crush this outbreak, and the more people we get vaccinated, the more assuredness that we’re going to have that we’re going to be able to do that,” Dr. Fauci said on CNN the month prior.
Dr. Memoli, who has studied influenza vaccination for years, disagreed, telling Dr. Fauci that research in the field has indicated yearly shots sometimes drive the evolution of influenza.
Vaccinating people who have not been infected with COVID-19, he said, could potentially impact the evolution of the virus that causes COVID-19 in unexpected ways.
“At best what we are doing with mandated mass vaccination does nothing and the variants emerge evading immunity anyway as they would have without the vaccine,” Dr. Memoli wrote. “At worst it drives evolution of the virus in a way that is different from nature and possibly detrimental, prolonging the pandemic or causing more morbidity and mortality than it should.”
The vaccination strategy was flawed because it relied on a single antigen, introducing immunity that only lasted for a certain period of time, Dr. Memoli said. When the immunity weakened, the virus was given an opportunity to evolve.
Some other experts, including virologist Geert Vanden Bossche, have offered similar views. Others in the scientific community, such as U.S. Centers for Disease Control and Prevention scientists, say vaccination prevents virus evolution, though the agency has acknowledged it doesn’t have records supporting its position.
Other Messages
Dr. Memoli sent the email to Dr. Fauci and two other top NIAID officials, Drs. Hugh Auchincloss and Clifford Lane. The message was first reported by the Wall Street Journal, though the publication did not publish the message. The Epoch Times obtained the email and 199 other pages of Dr. Memoli’s emails through a Freedom of Information Act request. There were no indications that Dr. Fauci ever responded to Dr. Memoli.
Later in 2021, the NIAID’s parent agency, the U.S. National Institutes of Health (NIH), and all other federal government agencies began requiring COVID-19 vaccination, under direction from President Joe Biden.
In other messages, Dr. Memoli said the mandates were unethical and that he was hopeful legal cases brought against the mandates would ultimately let people “make their own healthcare decisions.”
“I am certainly doing everything in my power to influence that,” he wrote on Nov. 2, 2021, to an unknown recipient. Dr. Memoli also disclosed that both he and his wife had applied for exemptions from the mandates imposed by the NIH and his wife’s employer. While her request had been granted, his had not as of yet, Dr. Memoli said. It’s not clear if it ever was.
According to Dr. Memoli, officials had not gone over the bioethics of the mandates. He wrote to the NIH’s Department of Bioethics, pointing out that the protection from the vaccines waned over time, that the shots can cause serious health issues such as myocarditis, or heart inflammation, and that vaccinated people were just as likely to spread COVID-19 as unvaccinated people.
He cited multiple studies in his emails, including one that found a resurgence of COVID-19 cases in a California health care system despite a high rate of vaccination and another that showed transmission rates were similar among the vaccinated and unvaccinated.
Dr. Memoli said he was “particularly interested in the bioethics of a mandate when the vaccine doesn’t have the ability to stop spread of the disease, which is the purpose of the mandate.”
The message led to Dr. Memoli speaking during an NIH event in December 2021, several weeks after he went public with his concerns about mandating vaccines.
“Vaccine mandates should be rare and considered only with a strong justification,” Dr. Memoli said in the debate. He suggested that the justification was not there for COVID-19 vaccines, given their fleeting effectiveness.
Julie Ledgerwood, another NIAID official who also spoke at the event, said that the vaccines were highly effective and that the side effects that had been detected were not significant. She did acknowledge that vaccinated people needed boosters after a period of time.
The NIH, and many other government agencies, removed their mandates in 2023 with the end of the COVID-19 public health emergency.
A request for comment from Dr. Fauci was not returned. Dr. Memoli told The Epoch Times in an email he was “happy to answer any questions you have” but that he needed clearance from the NIAID’s media office. That office then refused to give clearance.
Dr. Jay Bhattacharya, a professor of health policy at Stanford University, said that Dr. Memoli showed bravery when he warned Dr. Fauci against mandates.
“Those mandates have done more to demolish public trust in public health than any single action by public health officials in my professional career, including diminishing public trust in all vaccines.” Dr. Bhattacharya, a frequent critic of the U.S. response to COVID-19, told The Epoch Times via email. “It was risky for Dr. Memoli to speak publicly since he works at the NIH, and the culture of the NIH punishes those who cross powerful scientific bureaucrats like Dr. Fauci or his former boss, Dr. Francis Collins.”
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 International4 days ago
International4 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















