International
A next-generation therapeutic approach for patients after spinal cord injuries
NurExone (TSXV:NRX) has created an exosome-based drug-delivery platform and is developing a novel therapy for acute spinal cord injuries.
The post A next-generation…
Over the past two decades, exosomes – small, extracellular vesicles that are naturally released by many cell types and can carry a variety of molecular cargoes – have become the subject of increasingly intense scientific investigation. Studies suggest exosomes are important messengers for cells and organs, with as-yet unexplored diagnostic and therapeutic potential.
Today, NurExone Biologic Inc. (TSXV:NRX), headquartered in Haifa, Israel, is at the forefront of developing exosomes into next-generation nanocarriers for drug delivery. Drawing on deep expertise in exosome biology, NurExone has created the ExoTherapy technology platform, which comprises proprietary methods for the production, isolation and loading of molecules into exosomes for therapeutic purposes. NurExone is applying the ExoTherapy platform to create the company’s lead product, ExoPTEN, an intra-nasally administered exosome-based ExoTherapy to promote neuro-regeneration for the treatment of acute spinal cord injuries.
Harnessing the properties of exosomes for therapeutic applicationsMany cells produce extracellular vesicles (EVs), which are organized into subtypes with different sizes and biological functions. EVs generally fall into two categories: ectosomes, which pinch off from the cell membrane by outward budding; and much smaller exosomes, which have an endosomal origin and are created when endocytotic multivesicular bodies fuse with the plasma membrane, releasing the vesicles they contain as exosomes.
Scientific understanding of the origins and functions of exosomes is advancing, but there is widespread recognition that, far from being cellular waste products as once thought, exosomes play an important biological role in intercellular communication and transmission of macromolecules between cells. Further, through their cargo-carrying capacity, exosomes facilitate the spread of proteins, lipids, mRNA, miRNA, and DNA, which can contribute to their general therapeutic effects.
Beyond their normal biological roles, exosomes have increasingly gained attention as vehicles for the delivery of active pharmaceutical ingredients (APIs), from small molecules and peptides to proteins and nucleic acids, as an alternative not only to other kinds of nanocarriers such as lipid vesicles, but also cell-based gene therapies.
Exosomes offer a number of advantages as drug-delivery vehicles. As naturally occurring biological entities harvested from cells, exosomes have completely natural membranes that are better tolerated than many other types of drug-delivery vesicles synthesized from scratch in the laboratory. At the same time, exosomes do not seem to elicit the strong immune responses that often hamper allogeneic cell-based therapies used to deliver therapeutic molecules and genes to patients. In contrast to alternative therapeutic approaches, exosome therapies do not require expensive and time-consuming personalization, but can be used as “off-the-shelf” therapies suitable for all patients.
Exosomes have additional benefits as EV-based delivery vehicles for therapeutic agents. First, exosomes, including those produced by the ExoTherapy platform, can cross the blood–brain barrier (BBB), while other nanoparticles, such as most liposomes, cannot. ExoTherapy opens up the possibility of targeting different cell types – and, by extension, therapeutic indications – that are beyond the reach of non-BBB-crossing EVs1,2. Second, unmodified exosomes, even those carrying no molecular payload, have intrinsic properties that can be therapeutically beneficial, such as anti-inflammatory effects. Finally, exosomes can be administered intra-nasally.
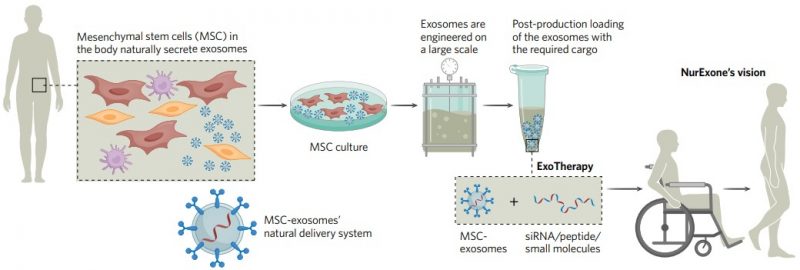
Exosomes originate from many sources. NurExone’s ExoTherapy platform employs exosomes derived from mesenchymal stem cells, which are effective in targeting neuronal cells. The ExoTherapy platform overcomes the many technical challenges involved in producing, purifying, and loading exosomes with APIs of almost any type (Fig. 1). Through its ability to carry a wide variety of therapeutic modalities, ExoTherapy stands as a true platform technology for creating “off-the-shelf” therapies that can be administered non-invasively.
Fig. 1 | From exosome to ExoTherapy: NurExone’s technology platform. NurExone is developing a platform for large-scale production of exosomes and loading of molecular cargo to create biologically guided ExoTherapy. The company’s vision is to restore motor function in patients after a spinal cord injury. siRNA, small interfering RNA. Development of a non-invasive therapy for functional recovery after SCIThe ExoTherapy platform sits at the heart of NurExone’s long-term business plan, providing a tool for creating a rich pipeline of novel therapeutic assets. In the near term, NurExone’s ambitious goal is to bring to market a novel treatment for acute spinal cord injuries (SCIs) derived from the ExoTherapy platform, ExoPTEN.
Globally, an estimated 250,000–500,000 people experience an SCI annually3, with roughly 17,000 new cases in the United States4 and 10,000 in Europe3 each year, bringing the potential market to ~50,000 new cases per year. Vehicular accidents and falls account for the majority of SCIs; sports and recreational accidents are another relatively common cause of SCI. Although the incidence of SCI is low compared with major disease such as cancer or heart disease, the effects are often devastating for patients, irreversible, and expensive to manage.
Depending on the location of the SCI, the consequences can be loss of sensory or motor control of both lower limbs (paraplegia), lower limbs and trunk, or both lower and upper limbs (tetraplegia). SCIs can also affect autonomic regulation of the body, affecting breathing, heart rate, blood pressure, temperature and bowel and bladder function.
Patients with an SCI typically spend almost two weeks in an intensive care unit, followed by a month in a rehabilitation unit. Fewer than 1% of people with an SCI experience full neurological recovery by the time of discharge, and have reduced quality of life and overall life expectancy4. SCI patients are also often frequently re-hospitalized, on average for almost three weeks, principally due to diseases of the genitourinary system, but also resulting from respiratory, circulatory and musculoskeletal problems.
In addition to the enormous physical toll SCIs exert on patients, they are also costly for health service providers. Depending on the location of the SCI, estimates place the cost of managing patients recovering from SCI at between $300,000 and >$1 million, representing a huge burden on health services and the families of new SCI patients.
There are two major obstacles to recovery from SCI. First is the poor innate regenerative capacity of the central nervous system. A major impediment to axonal growth is phosphatase and tensin homolog (PTEN), which downregulates the mammalian target of rapamycin (mTOR) activity and as a result restricts the synthesis of protein required for axonal growth. Second, SCI healing is hampered by the inflammation, myelin-associated inhibitors, glial scar components and compromised blood supply that typically surround SCIs and create a hostile environment for recovery.
ExoPTEN, which comprises exosomes loaded with small interfering RNA (siRNA) that inhibits the production of the PTEN protein, addresses both of these obstacles. The exosome component of ExoPTEN possesses intrinsic anti-inflammatory properties, which helps create a more hospitable recovery environment at the SCI site. Meanwhile, the anti-PTEN siRNA counters the suppressive effects of PTEN, activating downstream pathways necessary for the protein synthesis underlying axonal growth and regeneration (Fig. 2).
 Fig. 2 | ExoPTEN for the treatment of acute spinal cord injury. a, ExoPTEN: MSC-derived exosomes are loaded with PTEN siRNA (left). b, Non-invasive delivery. c, Mechanism of action: PTEN siRNA inhibits PTEN, downregulating PTEN-related pathways and promoting cell growth and proliferation. d, NurExone’s ambitious goal for ExoPTEN is to induce at least partial functional recovery in patients with acute spinal cord injuries. MSC, mesenchymal stem cell; PTEN, phosphatase and tensin homolog; siRNA, small interfering RNA.
Fig. 2 | ExoPTEN for the treatment of acute spinal cord injury. a, ExoPTEN: MSC-derived exosomes are loaded with PTEN siRNA (left). b, Non-invasive delivery. c, Mechanism of action: PTEN siRNA inhibits PTEN, downregulating PTEN-related pathways and promoting cell growth and proliferation. d, NurExone’s ambitious goal for ExoPTEN is to induce at least partial functional recovery in patients with acute spinal cord injuries. MSC, mesenchymal stem cell; PTEN, phosphatase and tensin homolog; siRNA, small interfering RNA.
ExoPTEN has been tested as an intra-nasally administered formulation in an extreme rat model of acute SCI: complete transection of the spinal cord resulting in paraplegia. In an internal preclinical study carried out by NurExone, untreated rats remained almost totally paralyzed eight weeks after surgical transection, whereas rats receiving ExoPTEN for a maximum of two weeks showed significant partial functional recovery. No ExoPTEN human trials have yet taken place but NurExone believes the therapy could translate to improvements in quality of life. (Unloaded exosomes also demonstrated a mild effect on post-operation recovery, highlighting the dual-effect nature of ExoPTEN.) ExoPTEN also partially restored healthy electrophysiological traces, indicative of axonal rewiring and regeneration, and improved sensory recovery and urinary reflex restoration.
ExoTherapy’s applications beyond spinal cord injuryThe regenerative effects observed with ExoPTEN in the severe spinal cord transection model suggest it may also have therapeutic applications in situations in which cell regeneration is a limiting factor for recovery. One major potential application of ExoPTEN identified by NurExone is traumatic brain injury, which affects more people than SCI and for which there are no effective pharmacological treatments that reduce mortality or improve functional recovery. Other potential therapeutic areas in which ExoPTEN may have a powerful impact include cardiac ischemia/reperfusion injury and associated disease, wound repair, and infertility.
While ExoPTEN employs exosomes to deliver siRNA, the ExoTherapy platform can just as easily be used to deliver other drug modalities. Moving forward, NurExone is planning to continue the development of in-house candidates such as ExoPTEN, and will also explore licensing possibilities for pharma companies looking for an enhanced delivery system for their drug(s) of various modalities, as well as opportunities to form partnerships and collaborations to jointly develop novel ExoTherapy-based medicines.
References Guo, S., Redenski, I. & Levenberg, S. Cells 10, 1872 (2021). Guo, S. et al. ACS Nano. 13, 10015–10028 (2019). World Health Organization. Factsheet: Spinal cord injury (2013). Available from: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury National Spinal Cord Injury Statistical Center, Facts and figures at a glance. Birmingham, AL: University of Birmingham at Alabama (2016).This is third-party provided content issued on behalf of NurExone Biologic, please see full disclaimer here.
Join the discussion: Find out what everybody’s saying about this stock on the NurExone Biologic Inc. Bullboard investor discussion forum, and check out the rest of Stockhouse’s stock forums and message boards.
The post A next-generation therapeutic approach for patients after spinal cord injuries appeared first on The Market Herald Canada.
treatment preclinical therapy rna dna recovery canada world health organizationInternational
Riley Gaines Explains How Women’s Sports Are Rigged To Promote The Trans Agenda
Riley Gaines Explains How Women’s Sports Are Rigged To Promote The Trans Agenda
Is there a light forming when it comes to the long, dark and…
Is there a light forming when it comes to the long, dark and bewildering tunnel of social justice cultism? Global events have been so frenetic that many people might not remember, but only a couple years ago Big Tech companies and numerous governments were openly aligned in favor of mass censorship. Not just to prevent the public from investigating the facts surrounding the pandemic farce, but to silence anyone questioning the validity of woke concepts like trans ideology.
From 2020-2022 was the closest the west has come in a long time to a complete erasure of freedom of speech. Even today there are still countries and Europe and places like Canada or Australia that are charging forward with draconian speech laws. The phrase "radical speech" is starting to circulate within pro-censorship circles in reference to any platform where people are allowed to talk critically. What is radical speech? Basically, it's any discussion that runs contrary to the beliefs of the political left.
Open hatred of moderate or conservative ideals is perfectly acceptable, but don't ever shine a negative light on woke activism, or you might be a terrorist.
Riley Gaines has experienced this double standard first hand. She was even assaulted and taken hostage at an event in 2023 at San Francisco State University when leftists protester tried to trap her in a room and demanded she "pay them to let her go." Campus police allegedly witnessed the incident but charges were never filed and surveillance footage from the college was never released.
It's probably the last thing a champion female swimmer ever expects, but her head-on collision with the trans movement and the institutional conspiracy to push it on the public forced her to become a counter-culture voice of reason rather than just an athlete.
For years the independent media argued that no matter how much we expose the insanity of men posing as women to compete and dominate women's sports, nothing will really change until the real female athletes speak up and fight back. Riley Gaines and those like her represent that necessary rebellion and a desperately needed return to common sense and reason.
In a recent interview on the Joe Rogan Podcast, Gaines related some interesting information on the inner workings of the NCAA and the subversive schemes surrounding trans athletes. Not only were women participants essentially strong-armed by colleges and officials into quietly going along with the program, there was also a concerted propaganda effort. Competition ceremonies were rigged as vehicles for promoting trans athletes over everyone else.
The bottom line? The competitions didn't matter. The real women and their achievements didn't matter. The only thing that mattered to officials were the photo ops; dudes pretending to be chicks posing with awards for the gushing corporate media. The agenda took precedence.
Lia Thomas, formerly known as William Thomas, was more than an activist invading female sports, he was also apparently a science project fostered and protected by the athletic establishment. It's important to understand that the political left does not care about female athletes. They do not care about women's sports. They don't care about the integrity of the environments they co-opt. Their only goal is to identify viable platforms with social impact and take control of them. Women's sports are seen as a vehicle for public indoctrination, nothing more.
The reasons why they covet women's sports are varied, but a primary motive is the desire to assert the fallacy that men and women are "the same" psychologically as well as physically. They want the deconstruction of biological sex and identity as nothing more than "social constructs" subject to personal preference. If they can destroy what it means to be a man or a woman, they can destroy the very foundations of relationships, families and even procreation.
For now it seems as though the trans agenda is hitting a wall with much of the public aware of it and less afraid to criticize it. Social media companies might be able to silence some people, but they can't silence everyone. However, there is still a significant threat as the movement continues to target children through the public education system and women's sports are not out of the woods yet.
The ultimate solution is for women athletes around the world to organize and widely refuse to participate in any competitions in which biological men are allowed. The only way to save women's sports is for women to be willing to end them, at least until institutions that put doctrine ahead of logic are made irrelevant.
International
Congress’ failure so far to deliver on promise of tens of billions in new research spending threatens America’s long-term economic competitiveness
A deal that avoided a shutdown also slashed spending for the National Science Foundation, putting it billions below a congressional target intended to…

Federal spending on fundamental scientific research is pivotal to America’s long-term economic competitiveness and growth. But less than two years after agreeing the U.S. needed to invest tens of billions of dollars more in basic research than it had been, Congress is already seriously scaling back its plans.
A package of funding bills recently passed by Congress and signed by President Joe Biden on March 9, 2024, cuts the current fiscal year budget for the National Science Foundation, America’s premier basic science research agency, by over 8% relative to last year. That puts the NSF’s current allocation US$6.6 billion below targets Congress set in 2022.
And the president’s budget blueprint for the next fiscal year, released on March 11, doesn’t look much better. Even assuming his request for the NSF is fully funded, it would still, based on my calculations, leave the agency a total of $15 billion behind the plan Congress laid out to help the U.S. keep up with countries such as China that are rapidly increasing their science budgets.
I am a sociologist who studies how research universities contribute to the public good. I’m also the executive director of the Institute for Research on Innovation and Science, a national university consortium whose members share data that helps us understand, explain and work to amplify those benefits.
Our data shows how underfunding basic research, especially in high-priority areas, poses a real threat to the United States’ role as a leader in critical technology areas, forestalls innovation and makes it harder to recruit the skilled workers that high-tech companies need to succeed.
A promised investment
Less than two years ago, in August 2022, university researchers like me had reason to celebrate.
Congress had just passed the bipartisan CHIPS and Science Act. The science part of the law promised one of the biggest federal investments in the National Science Foundation in its 74-year history.
The CHIPS act authorized US$81 billion for the agency, promised to double its budget by 2027 and directed it to “address societal, national, and geostrategic challenges for the benefit of all Americans” by investing in research.
But there was one very big snag. The money still has to be appropriated by Congress every year. Lawmakers haven’t been good at doing that recently. As lawmakers struggle to keep the lights on, fundamental research is quickly becoming a casualty of political dysfunction.
Research’s critical impact
That’s bad because fundamental research matters in more ways than you might expect.
For instance, the basic discoveries that made the COVID-19 vaccine possible stretch back to the early 1960s. Such research investments contribute to the health, wealth and well-being of society, support jobs and regional economies and are vital to the U.S. economy and national security.
Lagging research investment will hurt U.S. leadership in critical technologies such as artificial intelligence, advanced communications, clean energy and biotechnology. Less support means less new research work gets done, fewer new researchers are trained and important new discoveries are made elsewhere.
But disrupting federal research funding also directly affects people’s jobs, lives and the economy.
Businesses nationwide thrive by selling the goods and services – everything from pipettes and biological specimens to notebooks and plane tickets – that are necessary for research. Those vendors include high-tech startups, manufacturers, contractors and even Main Street businesses like your local hardware store. They employ your neighbors and friends and contribute to the economic health of your hometown and the nation.
Nearly a third of the $10 billion in federal research funds that 26 of the universities in our consortium used in 2022 directly supported U.S. employers, including:
A Detroit welding shop that sells gases many labs use in experiments funded by the National Institutes of Health, National Science Foundation, Department of Defense and Department of Energy.
A Dallas-based construction company that is building an advanced vaccine and drug development facility paid for by the Department of Health and Human Services.
More than a dozen Utah businesses, including surveyors, engineers and construction and trucking companies, working on a Department of Energy project to develop breakthroughs in geothermal energy.
When Congress shortchanges basic research, it also damages businesses like these and people you might not usually associate with academic science and engineering. Construction and manufacturing companies earn more than $2 billion each year from federally funded research done by our consortium’s members.

Jobs and innovation
Disrupting or decreasing research funding also slows the flow of STEM – science, technology, engineering and math – talent from universities to American businesses. Highly trained people are essential to corporate innovation and to U.S. leadership in key fields, such as AI, where companies depend on hiring to secure research expertise.
In 2022, federal research grants paid wages for about 122,500 people at universities that shared data with my institute. More than half of them were students or trainees. Our data shows that they go on to many types of jobs but are particularly important for leading tech companies such as Google, Amazon, Apple, Facebook and Intel.
That same data lets me estimate that over 300,000 people who worked at U.S. universities in 2022 were paid by federal research funds. Threats to federal research investments put academic jobs at risk. They also hurt private sector innovation because even the most successful companies need to hire people with expert research skills. Most people learn those skills by working on university research projects, and most of those projects are federally funded.
High stakes
If Congress doesn’t move to fund fundamental science research to meet CHIPS and Science Act targets – and make up for the $11.6 billion it’s already behind schedule – the long-term consequences for American competitiveness could be serious.
Over time, companies would see fewer skilled job candidates, and academic and corporate researchers would produce fewer discoveries. Fewer high-tech startups would mean slower economic growth. America would become less competitive in the age of AI. This would turn one of the fears that led lawmakers to pass the CHIPS and Science Act into a reality.
Ultimately, it’s up to lawmakers to decide whether to fulfill their promise to invest more in the research that supports jobs across the economy and in American innovation, competitiveness and economic growth. So far, that promise is looking pretty fragile.
This is an updated version of an article originally published on Jan. 16, 2024.
Jason Owen-Smith receives research support from the National Science Foundation, the National Institutes of Health, the Alfred P. Sloan Foundation and Wellcome Leap.
economic growth covid-19 grants congress vaccine chinaInternational
What’s Driving Industrial Development in the Southwest U.S.
The post-COVID-19 pandemic pipeline, supply imbalances, investment and construction challenges: these are just a few of the topics address by a powerhouse…

The post-COVID-19 pandemic pipeline, supply imbalances, investment and construction challenges: these are just a few of the topics address by a powerhouse panel of executives in industrial real estate this week at NAIOP’s I.CON West in Long Beach, California. Led by Dawn McCombs, principal and Denver lead industrial specialist for Avison Young, the panel tackled some of the biggest issues facing the sector in the Western U.S.
Starting with the pandemic in 2020 and continuing through 2022, McCombs said, the industrial sector experienced a huge surge in demand, resulting in historic vacancies, rent growth and record deliveries. Operating fundamentals began to normalize in 2023 and construction starts declined, certainly impacting vacancy and absorption moving forward.
“Development starts dropped by 65% year-over-year across the U.S. last year. In Q4, we were down 25% from pre-COVID norms,” began Megan Creecy-Herman, president, U.S. West Region, Prologis, noting that all of that is setting us up to see an improvement of fundamentals in the market. “U.S. vacancy ended 2023 at about 5%, which is very healthy.”
Vacancies are expected to grow in Q1 and Q2, peaking mid-year at around 7%. Creecy-Herman expects to see an increase in absorption as customers begin to have confidence in the economy, and everyone gets some certainty on what the Fed does with interest rates.
“It’s an interesting dynamic to see such a great increase in rents, which have almost doubled in some markets,” said Reon Roski, CEO, Majestic Realty Co. “It’s healthy to see a slowing down… before [rents] go back up.”
Pre-pandemic, a lot of markets were used to 4-5% vacancy, said Brooke Birtcher Gustafson, fifth-generation president of Birtcher Development. “Everyone was a little tepid about where things are headed with a mediocre outlook for 2024, but much of this is normalizing in the Southwest markets.”
McCombs asked the panel where their companies found themselves in the construction pipeline when the Fed raised rates in 2022.
In Salt Lake City, said Angela Eldredge, chief operations officer at Price Real Estate, there is a typical 12-18-month lead time on construction materials. “As rates started to rise in 2022, lots of permits had already been pulled and construction starts were beginning, so those project deliveries were in fall 2023. [The slowdown] was good for our market because it kept rates high, vacancies lower and helped normalize the market to a healthy pace.”
A supply imbalance can stress any market, and Gustafson joked that the current imbalance reminded her of a favorite quote from the movie Super Troopers: “Desperation is a stinky cologne.” “We’re all still a little crazed where this imbalance has put us, but for the patient investor and owner, there will be a rebalancing and opportunity for the good quality real estate to pass the sniff test,” she said.
At Bircher, Gustafson said that mid-pandemic, there were predictions that one billion square feet of new product would be required to meet tenant demand, e-commerce growth and safety stock. That transition opened a great opportunity for investors to run at the goal. “In California, the entitlement process is lengthy, around 24-36 months to get from the start of an acquisition to the completion of a building,” she said. Fast forward to 2023-2024, a lot of what is being delivered in 2024 is the result of that chase.
“Being an optimistic developer, there is good news. The supply imbalance helped normalize what was an unsustainable surge in rents and land values,” she said. “It allowed corporate heads of real estate to proactively evaluate growth opportunities, opened the door for contrarian investors to land bank as values drop, and provided tenants with options as there is more product. Investment goals and strategies have shifted, and that’s created opportunity for buyers.”
“Developers only know how to run and develop as much as we can,” said Roski. “There are certain times in cycles that we are forced to slow down, which is a good thing. In the last few years, Majestic has delivered 12-14 million square feet, and this year we are developing 6-8 million square feet. It’s all part of the cycle.”
Creecy-Herman noted that compared to the other asset classes and opportunities out there, including office and multifamily, industrial remains much more attractive for investment. “That was absolutely one of the things that underpinned the amount of investment we saw in a relatively short time period,” she said.
Market rent growth across Los Angeles, Inland Empire and Orange County moved up more than 100% in a 24-month period. That created opportunities for landlords to flexible as they’re filling up their buildings. “Normalizing can be uncomfortable especially after that kind of historic high, but at the same time it’s setting us up for strong years ahead,” she said.
Issues that owners and landlords are facing with not as much movement in the market is driving a change in strategy, noted Gustafson. “Comps are all over the place,” she said. “You have to dive deep into every single deal that is done to understand it and how investment strategies are changing.”
Tenants experienced a variety of challenges in the pandemic years, from supply chain to labor shortages on the negative side, to increased demand for products on the positive, McCombs noted.
“Prologis has about 6,700 customers around the world, from small to large, and the universal lesson [from the pandemic] is taking a more conservative posture on inventories,” Creecy-Herman said. “Customers are beefing up inventories, and that conservatism in the supply chain is a lesson learned that’s going to stick with us for a long time.” She noted that the company has plenty of clients who want to take more space but are waiting on more certainty from the broader economy.
“E-commerce grew by 8% last year, and we think that’s going to accelerate to 10% this year. This is still less than 25% of all retail sales, so the acceleration we’re going to see in e-commerce… is going to drive the business forward for a long time,” she said.
Roski noted that customers continually re-evaluate their warehouse locations, expanding during the pandemic and now consolidating but staying within one delivery day of vast consumer bases.
“This is a generational change,” said Creecy-Herman. “Millions of young consumers have one-day delivery as a baseline for their shopping experience. Think of what this means for our business long term to help our customers meet these expectations.”
McCombs asked the panelists what kind of leasing activity they are experiencing as a return to normalcy is expected in 2024.
“During the pandemic, shifts in the ports and supply chain created a build up along the Mexican border,” said Roski, noting border towns’ importance to increased manufacturing in Mexico. A shift of populations out of California and into Arizona, Nevada, Texas and Florida have resulted in an expansion of warehouses in those markets.
Eldridge said that Salt Lake City’s “sweet spot” is 100-200 million square feet, noting that the market is best described as a mid-box distribution hub that is close to California and Midwest markets. “Our location opens up the entire U.S. to our market, and it’s continuing to grow,” she said.
The recent supply chain and West Coast port clogs prompted significant investment in nearshoring and port improvements. “Ports are always changing,” said Roski, listing a looming strike at East Coast ports, challenges with pirates in the Suez Canal, and water issues in the Panama Canal. “Companies used to fix on one port and that’s where they’d bring in their imports, but now see they need to be [bring product] in a couple of places.”
“Laredo, [Texas,] is one of the largest ports in the U.S., and there’s no water. It’s trucks coming across the border. Companies have learned to be nimble and not focused on one area,” she said.
“All of the markets in the southwest are becoming more interconnected and interdependent than they were previously,” Creecy-Herman said. “In Southern California, there are 10 markets within 500 miles with over 25 million consumers who spend, on average, 10% more than typical U.S. consumers.” Combined with the port complex, those fundamentals aren’t changing. Creecy-Herman noted that it’s less of a California exodus than it is a complementary strategy where customers are taking space in other markets as they grow. In the last 10 years, she noted there has been significant maturation of markets such as Las Vegas and Phoenix. As they’ve become more diversified, customers want to have a presence there.
In the last decade, Gustafson said, the consumer base has shifted. Tenants continue to change strategies to adapt, such as hub-and-spoke approaches. From an investment perspective, she said that strategies change weekly in response to market dynamics that are unprecedented.
McCombs said that construction challenges and utility constraints have been compounded by increased demand for water and power.
“Those are big issues from the beginning when we’re deciding on whether to buy the dirt, and another decision during construction,” Roski said. “In some markets, we order transformers more than a year before they are needed. Otherwise, the time comes [to use them] and we can’t get them. It’s a new dynamic of how leases are structured because it’s something that’s out of our control.” She noted that it’s becoming a bigger issue with electrification of cars, trucks and real estate, and the U.S. power grid is not prepared to handle it.
Salt Lake City’s land constraints play a role in site selection, said Eldridge. “Land values of areas near water are skyrocketing.”
The panelists agreed that a favorable outlook is ahead for 2024, and today’s rebalancing will drive a healthy industry in the future as demand and rates return to normalized levels, creating opportunities for investors, developers and tenants.
This post is brought to you by JLL, the social media and conference blog sponsor of NAIOP’s I.CON West 2024. Learn more about JLL at www.us.jll.com or www.jll.ca.
fed pandemic covid-19 real estate interest rates mexico-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International5 days ago
International5 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International5 days ago
International5 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















