Uncategorized
US FDA grants regular approval for Jemperli for the treatment of patients with recurrent or advanced mismatch repair-deficient endometrial cancer
US FDA grants regular approval for Jemperli for the treatment of patients with recurrent or advanced mismatch repair-deficient endometrial cancer
PR Newswire
LONDON, Feb. 10, 2023
Conversion from accelerated to regular (full) approval based on long…

US FDA grants regular approval for Jemperli for the treatment of patients with recurrent or advanced mismatch repair-deficient endometrial cancer
PR Newswire
LONDON, Feb. 10, 2023
- Conversion from accelerated to regular (full) approval based on long-term outcomes from the GARNET phase I trial, which demonstrated an overall response rate of 45.4%
- 85.9% of patients had duration of response ≥12 months and 54.7% of patients had duration of response ≥24 months
LONDON, Feb. 10, 2023 /PRNewswire/ -- GSK plc (LSE/NYSE: GSK) today reports that the US Food and Drug Administration (FDA) granted full approval for Jemperli (dostarlimab-gxly) for the treatment of adult patients with mismatch repair-deficient (dMMR) recurrent or advanced endometrial cancer, as determined by a US FDA-approved test, that has progressed on or following a prior platinum-containing regimen in any setting and are not candidates for curative surgery or radiation.
Hesham Abdullah, Senior Vice President, Global Head of Oncology Development, GSK, said: "This US regulatory action confirms our confidence in Jemperli as an important treatment option for patients with dMMR recurrent or advanced endometrial cancer. We continue to unlock the potential of Jemperli as the backbone for our immuno-oncology development programs to address the unmet needs of patients, including earlier lines of endometrial cancer and other solid tumors."
In April 2021, Jemperli received accelerated approval for the treatment of adult patients with dMMR recurrent or advanced endometrial cancer that had progressed on or following prior treatment with a platinum-containing regimen.
This approval is based on additional data collected from the A1 expansion cohort of the ongoing GARNET trial, a phase I, multicenter, open-label, single-arm study of Jemperli monotherapy in patients with advanced or recurrent solid tumors. Cohort A1 evaluated the efficacy of Jemperli in 141 patients with dMMR advanced or recurrent endometrial cancer that has progressed on or following prior treatment with a platinum-containing regimen. The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR) as assessed by a blinded independent central review according to RECIST v1.1. Confirmed ORR was 45.4% (95% CI: 37.0, 54.0), with a 15.6% complete response rate and a 29.8% partial response rate. Median DOR was not reached (range: 1.2+, 52.8+ months), measured from the time of first response, with 85.9% of patients having duration ≥12 months and 54.7% of patients having duration ≥24 months. Median follow-up for duration of response was 27.9 months.
Treatment-related adverse events were consistent with previous analyses for cohort A1. The most common adverse reactions (≥20%) were fatigue/asthenia, anemia, rash, nausea, diarrhea, constipation, and vomiting. The most common Grade 3 or 4 adverse reactions (≥2%) were anemia, increased transaminases, urinary tract infection, fatigue/asthenia, and diarrhea.
About endometrial cancer
Endometrial cancer is found in the inner lining of the uterus, known as the endometrium. Endometrial cancer is the most common gynecologic cancer globally, with approximately 417,000 new cases reported each year worldwide[1], and incidence rates are expected to rise by almost 40% by 2040.[2][3] Approximately 15-20% of patients with endometrial cancer will be diagnosed with advanced disease at the time of diagnosis.[4]
About GARNET
The ongoing GARNET phase I trial is evaluating Jemperli as monotherapy in patients with advanced solid tumors. Part 2B of the study includes five expansion cohorts: dMMR/MSI-H endometrial cancer (cohort A1), mismatch repair proficient/microsatellite stable (MMRp/MSS) endometrial cancer (cohort A2), non-small cell lung cancer (cohort E), dMMR/MSI-H non-endometrial or POLE-mut solid tumor basket cohort (cohort F), and platinum-resistant ovarian cancer without BRCA mutations (cohort G). GARNET continues to enroll patients.
In cohort A1, patients received 500mg of Jemperli as an intravenous infusion once every three weeks (Q3W) for four doses, followed by 1,000mg once every six weeks until disease progression, discontinuation or withdrawal.
About Jemperli (dostarlimab-gxly)
Jemperli is a programmed death receptor-1 (PD-1)-blocking antibody that binds to the PD-1 receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2.[5] GSK's ambition is for Jemperli to become the backbone of the Company's ongoing immuno-oncology-based research and development program when used alone and in combination with standard of care and future novel cancer therapies, particularly for patients who currently have limited treatment options. Jemperli is being investigated in registrational enabling studies as monotherapy and as part of combination regimens, including in patients with recurrent or primary advanced endometrial cancer, patients with Stage III or IV non-mucinous epithelial ovarian cancer, and patients with other advanced solid tumors or metastatic cancers.
Jemperli was discovered by AnaptysBio, Inc. and licensed to TESARO, Inc., under a collaboration and exclusive license agreement signed in March 2014. The collaboration has resulted in three monospecific antibody therapies that have progressed into the clinic. These are: Jemperli (GSK4057190), a PD-1 antagonist; cobolimab, (GSK4069889), a TIM-3 antagonist; and GSK4074386, a LAG-3 antagonist. GSK is responsible for the ongoing research, development, commercialization, and manufacturing of each of these medicines under the agreement.
Indications and Important Safety Information for JEMPERLI (dostarlimab-gxly)
JEMPERLI is indicated for the treatment of adult patients with mismatch repair deficient (dMMR) recurrent or advanced:
- endometrial cancer (EC), as determined by an FDA-approved test, that has progressed on or following prior treatment with a platinum-containing regimen in any setting and are not candidates for curative surgery or radiation, or
- solid tumors, as determined by an FDA-approved test, that have progressed on or following prior treatment and who have no satisfactory alternative treatment options. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
- Immune-mediated adverse reactions, which can be severe or fatal, can occur in any organ system or tissue and can occur at any time during or after treatment with a PD-1/PD-L1–blocking antibody, including JEMPERLI.
- Monitor closely for signs and symptoms of immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function tests at baseline and periodically during treatment. For suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
- Based on the severity of the adverse reaction, withhold or permanently discontinue JEMPERLI. In general, if JEMPERLI requires interruption or discontinuation, administer systemic corticosteroids (1 to 2 mg/kg/day prednisone or equivalent) until improvement to ≤Grade 1. Upon improvement to ≤Grade 1, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reaction is not controlled with corticosteroids.
Immune-Mediated Pneumonitis
- JEMPERLI can cause immune-mediated pneumonitis, which can be fatal. In patients treated with other PD-1/PD-L1–blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Pneumonitis occurred in 2.3% (14/605) of patients, including Grade 2 (1.3%), Grade 3 (0.8%), and Grade 4 (0.2%) pneumonitis.
Immune-Mediated Colitis
- Colitis occurred in 1.3% (8/605) of patients, including Grade 2 (0.7%) and Grade 3 (0.7%) adverse reactions. Cytomegalovirus infection/reactivation have occurred in patients with corticosteroid-refractory immune-mediated colitis. In such cases, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
- JEMPERLI can cause immune-mediated hepatitis, which can be fatal. Grade 3 hepatitis occurred in 0.5% (3/605) of patients.
Immune-Mediated Endocrinopathies
- Adrenal Insufficiency
- Adrenal insufficiency occurred in 1.2% (7/605) of patients, including Grade 2 (0.5%) and Grade 3 (0.7%). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment per institutional guidelines, including hormone replacement as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity.
- Hypophysitis
- JEMPERLI can cause immune-mediated hypophysitis. Grade 2 hypophysitis occurred in 0.2% (1/605) of patients. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity.
- Thyroid Disorders
- Grade 2 thyroiditis occurred in 0.5% (3/605) of patients. Grade 2 hypothyroidism occurred in 7.6% (46/605) of patients. Hyperthyroidism occurred in 2.3% (14/605) of patients, including Grade 2 (2.1%) and Grade 3 (0.2%). Initiate hormone replacement or medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity.
- Type 1 Diabetes Mellitus, Which Can Present with Diabetic Ketoacidosis
- JEMPERLI can cause type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Grade 3 type 1 diabetes mellitus occurred in 0.2% (1/605) of patients. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity.
Immune-Mediated Nephritis with Renal Dysfunction
- JEMPERLI can cause immune-mediated nephritis, which can be fatal. Grade 2 nephritis, including tubulointerstitial nephritis, occurred in 0.5% (3/605) of patients.
Immune-Mediated Dermatologic Adverse Reactions
- JEMPERLI can cause immune-mediated rash or dermatitis. Bullous and exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS), have occurred with PD-1/PD-L1–blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes. Withhold or permanently discontinue JEMPERLI depending on severity.
Other Immune-Mediated Adverse Reactions
- The following clinically significant immune-mediated adverse reactions occurred in <1% of the 605 patients treated with JEMPERLI or were reported with the use of other PD-1/PD-L1–blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
- Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis, Guillain-Barre syndrome, nerve paresis, autoimmune neuropathy
- Cardiac/Vascular: Myocarditis, pericarditis, vasculitis
- Ocular: Uveitis, iritis, other ocular inflammatory toxicities. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur
- Gastrointestinal: Pancreatitis, including increases in serum amylase and lipase levels, gastritis, duodenitis
- Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatica
- Endocrine: Hypoparathyroidism
- Other (Hematologic/Immune): Autoimmune hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection
Infusion-Related Reactions
- Severe or life-threatening infusion-related reactions have been reported with PD-1/PD-L1–blocking antibodies. Severe infusion-related reactions (Grade 3) occurred in 0.2% (1/605) of patients receiving JEMPERLI. Monitor patients for signs and symptoms of infusion-related reactions. Interrupt or slow the rate of infusion or permanently discontinue JEMPERLI based on severity of reaction.
Complications of Allogeneic HSCT
- Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after treatment with a PD-1/PD-L1–blocking antibody, which may occur despite intervening therapy. Monitor patients closely for transplant-related complications and intervene promptly.
Embryo-Fetal Toxicity and Lactation
- Based on its mechanism of action, JEMPERLI can cause fetal harm. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with JEMPERLI and for 4 months after their last dose. Because of the potential for serious adverse reactions from JEMPERLI in a breastfed child, advise women not to breastfeed during treatment with JEMPERLI and for 4 months after their last dose.
Common Adverse Reactions
The most common adverse reactions (≥20%) in patients with dMMR EC were fatigue/asthenia, anemia, rash, nausea, diarrhea, constipation, and vomiting. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased lymphocytes, decreased sodium, increased alanine aminotransferase, increased creatinine, decreased neutrophils, decreased albumin, and increased alkaline phosphatase.
The most common adverse reactions (≥20%) in patients with dMMR solid tumors were fatigue/asthenia, anemia, diarrhea, and nausea. The most common Grade 3 or 4 laboratory abnormalities (≥2%) were decreased lymphocytes, decreased sodium, increased alkaline phosphatase, and decreased albumin.
Please see the full US Prescribing Information.
GSK in oncology
GSK is committed to maximizing patient survival through transformational medicines. GSK's pipeline is focused on immuno-oncology, tumor cell targeting therapies and synthetic lethality. Our goal is to achieve a sustainable flow of new treatments based on a diversified portfolio of investigational medicines utilizing modalities such as small molecules, antibodies, and antibody-drug conjugates, either alone or in combination.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at us.gsk.com/en-us/company.
GSK inquiries | |||
Media: | Tim Foley | +44 (0) 20 8047 5502 | (London) |
Dan Smith | +44 (0) 20 8047 5502 | (London) | |
Kathleen Quinn | +1 202 603 5003 | (Washington DC) | |
Lyndsay Meyer | +1 202 302 4595 | (Washington DC) | |
Investor Relations: | Nick Stone | +44 (0) 7717 618834 | (London) |
James Dodwell | +44 (0) 20 8047 2406 | (London) | |
Mick Readey | +44 (0) 7990 339653 | (London) | |
Josh Williams | +44 (0) 7385 415719 | (London) | |
Camilla Campbell | +44 (0) 7803 050238 | (London) | |
Steph Mountifield | +44 (0) 7736 063933 | (London) | |
Jeff McLaughlin | +1 215 751 7002 | (Philadelphia) | |
Frannie DeFranco | +1 215 751 4855 | (Philadelphia) |
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the Company's Annual Report on Form 20-F for 2021, GSK's Q4 Results for 2022 and any impacts of the COVID-19 pandemic.
Registered in England & Wales:
No. 3888792
Registered Office:
980 Great West Road
Brentford, Middlesex
TW8 9GS
References
1 Sung H, Ferlay J, Siegel R et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. doi:10.3322/caac.21660.
2 Braun MM, et al. Am Fam Physician. 2016;93(6):468-474.
3 International Research on Cancer. Global Cancer Observatory. Cancer Tomorrow. https://gco.iarc.fr/tomorrow/en/dataviz/. Accessed 13 July 2022.
4 Kantar Health, Cust Study (2018).
5 Laken H, Kehry M, Mcneeley P, et al. Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody. European Journal of Cancer. 2016;69,S102. doi:10.1016/s0959-8049(16)32902-1.
View original content to download multimedia:https://www.prnewswire.com/news-releases/us-fda-grants-regular-approval-for-jemperli-for-the-treatment-of-patients-with-recurrent-or-advanced-mismatch-repair-deficient-endometrial-cancer-301743848.html
SOURCE GSK plc
Uncategorized
One city held a mass passport-getting event
A New Orleans congressman organized a way for people to apply for their passports en masse.

While the number of Americans who do not have a passport has dropped steadily from more than 80% in 1990 to just over 50% now, a lack of knowledge around passport requirements still keeps a significant portion of the population away from international travel.
Over the four years that passed since the start of covid-19, passport offices have also been dealing with significant backlog due to the high numbers of people who were looking to get a passport post-pandemic.
Related: Here is why it is (still) taking forever to get a passport
To deal with these concurrent issues, the U.S. State Department recently held a mass passport-getting event in the city of New Orleans. Called the "Passport Acceptance Event," the gathering was held at a local auditorium and invited residents of Louisiana’s 2nd Congressional District to complete a passport application on-site with the help of staff and government workers.
'Come apply for your passport, no appointment is required'
"Hey #LA02," Rep. Troy A. Carter Sr. (D-LA), whose office co-hosted the event alongside the city of New Orleans, wrote to his followers on Instagram (META) . "My office is providing passport services at our #PassportAcceptance event. Come apply for your passport, no appointment is required."
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
The event was held on March 14 from 10 a.m. to 1 p.m. While it was designed for those who are already eligible for U.S. citizenship rather than as a way to help non-citizens with immigration questions, it helped those completing the application for the first time fill out forms and make sure they have the photographs and identity documents they need. The passport offices in New Orleans where one would normally have to bring already-completed forms have also been dealing with lines and would require one to book spots weeks in advance.
These are the countries with the highest-ranking passports in 2024
According to Carter Sr.'s communications team, those who submitted their passport application at the event also received expedited processing of two to three weeks (according to the State Department's website, times for regular processing are currently six to eight weeks).
While Carter Sr.'s office has not released the numbers of people who applied for a passport on March 14, photos from the event show that many took advantage of the opportunity to apply for a passport in a group setting and get expedited processing.
Every couple of months, a new ranking agency puts together a list of the most and least powerful passports in the world based on factors such as visa-free travel and opportunities for cross-border business.
In January, global citizenship and financial advisory firm Arton Capital identified United Arab Emirates as having the most powerful passport in 2024. While the United States topped the list of one such ranking in 2014, worsening relations with a number of countries as well as stricter immigration rules even as other countries have taken strides to create opportunities for investors and digital nomads caused the American passport to slip in recent years.
A UAE passport grants holders visa-free or visa-on-arrival access to 180 of the world’s 198 countries (this calculation includes disputed territories such as Kosovo and Western Sahara) while Americans currently have the same access to 151 countries.
stocks pandemic covid-19 grantsUncategorized
Fast-food chain closes restaurants after Chapter 11 bankruptcy
Several major fast-food chains recently have struggled to keep restaurants open.

Competition in the fast-food space has been brutal as operators deal with inflation, consumers who are worried about the economy and their jobs and, in recent months, the falling cost of eating at home.
Add in that many fast-food chains took on more debt during the covid pandemic and that labor costs are rising, and you have a perfect storm of problems.
It's a situation where Restaurant Brands International (QSR) has suffered as much as any company.
Related: Wendy's menu drops a fan favorite item, adds something new
Three major Burger King franchise operators filed for bankruptcy in 2023, and the chain saw hundreds of stores close. It also saw multiple Popeyes franchisees move into bankruptcy, with dozens of locations closing.
RBI also stepped in and purchased one of its key franchisees.
"Carrols is the largest Burger King franchisee in the United States today, operating 1,022 Burger King restaurants in 23 states that generated approximately $1.8 billion of system sales during the 12 months ended Sept. 30, 2023," RBI said in a news release. Carrols also owns and operates 60 Popeyes restaurants in six states."
The multichain company made the move after two of its large franchisees, Premier Kings and Meridian, saw multiple locations not purchased when they reached auction after Chapter 11 bankruptcy filings. In that case, RBI bought select locations but allowed others to close.
Image source: Chen Jianli/Xinhua via Getty
Another fast-food chain faces bankruptcy problems
Bojangles may not be as big a name as Burger King or Popeye's, but it's a popular chain with more than 800 restaurants in eight states.
"Bojangles is a Carolina-born restaurant chain specializing in craveable Southern chicken, biscuits and tea made fresh daily from real recipes, and with a friendly smile," the chain says on its website. "Founded in 1977 as a single location in Charlotte, our beloved brand continues to grow nationwide."
Like RBI, Bojangles uses a franchise model, which makes it dependent on the financial health of its operators. The company ultimately saw all its Maryland locations close due to the financial situation of one of its franchisees.
Unlike. RBI, Bojangles is not public — it was taken private by Durational Capital Management LP and Jordan Co. in 2018 — which means the company does not disclose its financial information to the public.
That makes it hard to know whether overall softness for the brand contributed to the chain seeing its five Maryland locations after a Chapter 11 bankruptcy filing.
Bojangles has a messy bankruptcy situation
Even though the locations still appear on the Bojangles website, they have been shuttered since late 2023. The locations were operated by Salim Kakakhail and Yavir Akbar Durranni. The partners operated under a variety of LLCs, including ABS Network, according to local news channel WUSA9.
The station reported that the owners face a state investigation over complaints of wage theft and fraudulent W2s. In November Durranni and ABS Network filed for bankruptcy in New Jersey, WUSA9 reported.
"Not only do former employees say these men owe them money, WUSA9 learned the former owners owe the state, too, and have over $69,000 in back property taxes."
Former employees also say that the restaurant would regularly purchase fried chicken from Popeyes and Safeway when it ran out in their stores, the station reported.
Bojangles sent the station a comment on the situation.
"The franchisee is no longer in the Bojangles system," the company said. "However, it is important to note in your coverage that franchisees are independent business owners who are licensed to operate a brand but have autonomy over many aspects of their business, including hiring employees and payroll responsibilities."
Kakakhail and Durranni did not respond to multiple requests for comment from WUSA9.
bankruptcy pandemicUncategorized
Industrial Production Increased 0.1% in February
From the Fed: Industrial Production and Capacity Utilization
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 p…
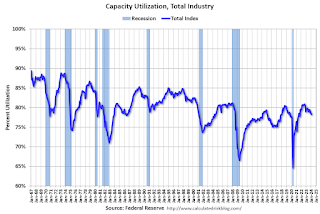
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 percent. Both gains partly reflected recoveries from weather-related declines in January. The index for utilities fell 7.5 percent in February because of warmer-than-typical temperatures. At 102.3 percent of its 2017 average, total industrial production in February was 0.2 percent below its year-earlier level. Capacity utilization for the industrial sector remained at 78.3 percent in February, a rate that is 1.3 percentage points below its long-run (1972–2023) average.Click on graph for larger image.
emphasis added
This graph shows Capacity Utilization. This series is up from the record low set in April 2020, and above the level in February 2020 (pre-pandemic).
Capacity utilization at 78.3% is 1.3% below the average from 1972 to 2022. This was below consensus expectations.
Note: y-axis doesn't start at zero to better show the change.
 The second graph shows industrial production since 1967.
The second graph shows industrial production since 1967.Industrial production increased to 102.3. This is above the pre-pandemic level.
Industrial production was above consensus expectations.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International1 week ago
International1 week agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 week ago
International1 week agoWalmart launches clever answer to Target’s new membership program
-

 Spread & Containment3 days ago
Spread & Containment3 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex



















