SomnoMed posts record Q4 and delivers on FY22 revenue guidance
SomnoMed posts record Q4 and delivers on FY22 revenue guidance
PR Newswire
PLANO, Texas, July 26, 2022
PLANO, Texas, July 26, 2022 /PRNewswire/ — SomnoMed Limited (ASX “SOM”, or the Company), a leading company in the provision of oral appliance tr…

SomnoMed posts record Q4 and delivers on FY22 revenue guidance
PR Newswire
PLANO, Texas, July 26, 2022
PLANO, Texas, July 26, 2022 /PRNewswire/ -- SomnoMed Limited (ASX "SOM", or the Company), a leading company in the provision of oral appliance treatment solutions for sleep-related breathing disorders and obstructive sleep apnea ('OSA'), is pleased to provide its quarterly activities report for the period ended 30 June 2022 (Q4 FY22).
Highlights
- Record quarterly revenue of $21.3 million for Q4 FY22, +32% (+32% in constant currency) versus the previous corresponding period (pcp). On an underlying basis, when excluding the Q4 FY21 COVID-19 related HIC1 allowance, Q4 FY22 revenue was up +36% (+37% in constant currency) versus pcp
- Full year unaudited revenue of $72.6 million, up +16% (+17% in constant currency) versus pcp and above guidance of +15% growth for FY22. On an underlying basis, when excluding the COVID-19 related HIC1 allowance, FY22 revenue was up +19% (+20% in constant currency) versus pcp
- FY22 guidance of breakeven EBITDA2 remains
- The record quarter reflects the impact of successful sales and marketing initiatives in all regions, with business conditions improving and the overall trend of patient activity and engagement with their medical clinician normalizing post COVID-19
- Total patients treated worldwide now exceeds 715,000
- Development of Rest Assure®, the Company's first ever in-built technology-enabled oral appliance progressed during the quarter, with the second patient validation study now complete
- SomnoMed had available cash of $15.6 million as at 30 June 2022
- Subsequent to 30 June 2022, SomnoMed secured new debt funding of $16 million (net $11m after repaying HSBC), which delivers sufficient capital to support the ongoing growth within the core business and to complete the development of the new technology, Rest Assure®
Commenting on the results, SomnoMed's Managing Director, Mr. Neil Verdal-Austin said: "I am exceptionally pleased that the Company has met our FY22 revenue guidance and immensely proud that we delivered a record $21.3 million of revenue in the June quarter."
"The improving sales levels SomnoMed is experiencing have been driven by a combination of the strong underlying business activity levels across our key markets and the results of the deliberate investments we made through 2021 to expand our sales and marketing teams to focus on delivering a quality treatment solution for patients with mild to moderate OSA. The sales momentum we have achieved over recent quarters continues to demonstrate the sound fundamentals of the core business and the large growth opportunity for oral appliance solutions in the treatment of obstructive sleep apnea, globally."
"These results validate our strategy and confirm, once again, that the exceptional 'first time fit' quality and durability of our product range is superior within the marketplace and preferred by clinicians and patients world-wide."
"Our recent attendance at a range of sleep conferences across Europe and the USA have highlighted the growing demand from patients, sleep physicians and other providers, for new technologies that provide an alternative to CPAP where the ongoing, low compliance rates are just not acceptable anymore."
"We are also acutely aware of the current global economic environment and the impact of supply chain issues, rising inflation and interest rates on costs. SomnoMed continues to proactively drive a range of initiatives to secure our supply chain and ensure we limit any negative impact on our own cost structures, so that we protect margins and cash flow."
"The development of SomnoMed's Rest Assure® technology has progressed during the quarter, with our second patient validation study now complete. We anticipate that, once launched and over time, the data collected by Rest Assure® will demonstrate and establish the true clinical effectiveness of Continuous Open Airway Therapy™ (COAT™), driving prescriptions, increased reimbursement and ultimately greater OSA therapy market share."
"As we move into FY23 we have strong revenue momentum, an increasing rate of patient engagement, a passionate and energized global team, a strengthened balance sheet and an exciting technology development in Rest Assure®, which all leave the Company poised to deliver solid growth in the year ahead. I look forward to providing more formal FY23 guidance with our FY22 financial results release in August."
Financial Review
Quarterly revenue of $21.3 million for Q4 FY22, +32% (+32% in constant currency) versus pcp represents the highest quarterly revenue the Company has ever recorded. This reflects the sales success of both AVANT™ and Herbst Advance Elite™ and the investment the Company has made into sales teams and business development channels in all regions, allowing the Company to penetrate the sector more deeply and drive growth.
The revenue growth posted in both North America and Europe continued, with the momentum seen in those regions during the year driven by the continued normalization of the impacts of COVID-19 on the healthcare market.
Revenue (A$000's) | Q4 FY22 | Q4 FY21 | % Change |
(A$000's) | (A$000's) | ||
Europe | 12,558 | 9,178 | +37 % |
North America | 7,057 | 4,955 | +42 % |
APAC | 1,436 | 1,335 | +8 % |
Total regional revenue | 21,051 | 15,468 | +36 % |
HIC1 | 251 | 700 | -64 % |
Total group revenue | 21,303 | 16,169 | +32 % |
Full year unaudited revenue of $72.6 million, up +16% (+17% in constant currency) versus pcp and above guidance for FY22. EBITDA2 guidance remains at breakeven for FY22 and will be detailed in the upcoming FY22 financial results release in August.
Revenue (A$000's) | 12 months to | 12 months to | % Change |
(A$000's) | (A$000's) | ||
Europe | 42,214 | 36,769 | +15 % |
North America | 24,688 | 18,506 | +33 % |
APAC | 5,427 | 5,369 | 1 % |
Total regional revenue | 72,329 | 60,644 | +19 % |
HIC1 | 251 | 2,062 | -88 % |
Total group revenue | 72,580 | 62,706 | +16 % |
Cashflow from operations was a positive $3.3 million for the quarter (positive $1.8 million for FY22), due to the strong sales momentum seen in both Q3 and Q4, as well as effective cash management and collections to year end.
Investment in R&D continues with total cash expenditure during the quarter of $1.5 million specifically related to our technology investment. The FY22 technology investment has been approximately $7.8 million, primarily associated with the development of Rest Assure® and under the guidance provided of $8 million. The investment in Rest Assure® and our other growth initiatives is supported by our balance sheet position and positive cash generation in Q4 FY22.
Subsequent to June 2022, SomnoMed secured new debt funding of $16 million (net $11m after repaying HSBC), which delivers sufficient capital to support the ongoing growth within the core business and to complete the development of the new technology, Rest Assure®.
Operational Review
Business conditions continued to improve across SomnoMed's key regions of North America, Europe and Asia Pacific as the impacts of COVID-19 on the medical sector and broader dental community continued to reduce.
SomnoMed's position in the oral appliance sector within the OSA market remains strong, with the potential to further increase the addressable market by providing an alternative to the traditional default CPAP recommendation by most sleep physicians. The Company continues to experience positive engagement within the medical sector, which is driving further acceptance of COAT™.
North America
The North America market experienced another positive quarter with a +42% (+33% in constant currency) revenue increase versus pcp. This is driven by increased investment in sales and marketing efforts in the region driving greater demand for the product range, especially for the AVANT™ and Herbst Advance Elite™. Direct and targeted marketing campaigns focused on the Company's proprietary B-Flex comfort liner continue to be successful, highlighting the importance of this material that provides both superior comfort and retention.
Europe
Revenue for the quarter was $12.6 million, up +37% (+43% in constant currency) versus pcp. Patient demand for the Company's COAT™ technology remains strong across core countries within Europe driven by strong positive reimbursement trends and a growing acceptance of the benefits of COAT™ technology for mild and moderate OSA patients.
Sales volume growth for the quarter in each of the 7 main European countries in which we trade were over +20% versus volumes in the prior corresponding quarter, reflecting deeper penetration into both core and new markets where SomnoMed has been the leading COAT™ treatment solution for some time.
Asia Pacific
Asia Pacific quarterly revenues were up +8% (+8% in constant currency) versus pcp. After the extended impact of COVID-19 on revenues in the first half, recovery in the second half was strong. SomnoMed continued with its clinical education program and the investment in new sales and marketing resources to advance the adoption of oral appliances within the medical sector.
Rest Assure®
SomnoMed introduced Rest Assure®, its first ever in-built technology-enabled oral appliance, in February 2022 with the aim of addressing the lack of overnight monitoring and objective data in COAT™, which has been a major barrier to prescription and reimbursement rates to date.
Although the Rest Assure® hardware and software is in prototype stage, the design is now complete, and a vendor is manufacturing the docking station and sensor components. The second patient validation study has also been completed. These study results are currently being analyzed and will be submitted for publication in a scientific sleep journal later this calendar year to confirm the algorithms needed to objectively measure efficacy and compliance to ensure long term therapy effectiveness. Patient feedback was also collected during this study, with 29/30 patients able to connect their device to the patient App and dock their device using simple written instructions only, showing the Rest Assure® system is easy to use. All patients in the study expressed a desire to continue COAT™ therapy with Rest Assure® when the device is launched.
The Rest Assure® project remains on schedule, with SomnoMed's focus now on the preparation of documentation required for regulatory submissions to the FDA (USA), TGA (Australia) and for CE marking (Europe). Rest Assure® will be commercialized once these approvals are received.
The Company attended the American Academy of Sleep Medicine (AASM) 'SLEEP 2022' conference in Charlotte USA during the quarter. AASM SLEEP 2022 is a leading conference for sleep physicians, sleep medical prescribers and researchers in North America focusing on all aspects of sleep, sleep disorders, screening, pharmaceuticals, technology platforms, tele-medicine, diagnostics, and treatment. The conference with approximately 3,500 delegates allowed the Company to showcase its best-in-class oral appliance treatment solution while also engaging in a range of collaborative interactions and meetings with various industry players. The feedback on the Rest Assure® technology was overwhelmingly positive.
This release has been approved by the Board of SomnoMed Limited.
For further information please contact
Corporate Mr. Neil Verdal-Austin CEO SomnoMed +61 406 931 477 | Investors Mr. Craig Sainsbury Market Eye +61 428 550 499 |
About SomnoMed
SomnoMed is a public company providing treatment solutions for sleep-related breathing disorders including obstructive sleep apnea, snoring and bruxism. SomnoMed was commercialised on the basis of extensive clinical research. Supporting independent clinical research, continuous innovation and instituting medical manufacturing standards has resulted in SomnoDent® becoming the state-of-the-art and clinically proven medical oral appliance therapy for more than 715,000 patients in 28 countries. For additional information, visit SomnoMed at http://www.somnomed.com.au
________________________ | |
1 | HIC (Health Care Companies) Revenue is associated with allowances received in the Netherlands which compensates SOM for a portion of lost managed care income in the country due to COVID-19 |
2 | EBITDA as adjusted does not include share-based payments, discontinued operations, and other expenses |
View original content to download multimedia:https://www.prnewswire.com/news-releases/somnomed-posts-record-q4-and-delivers-on-fy22-revenue-guidance-301593696.html
SOURCE SomnoMed
Uncategorized
Homes listed for sale in early June sell for $7,700 more
New Zillow research suggests the spring home shopping season may see a second wave this summer if mortgage rates fall
The post Homes listed for sale in…
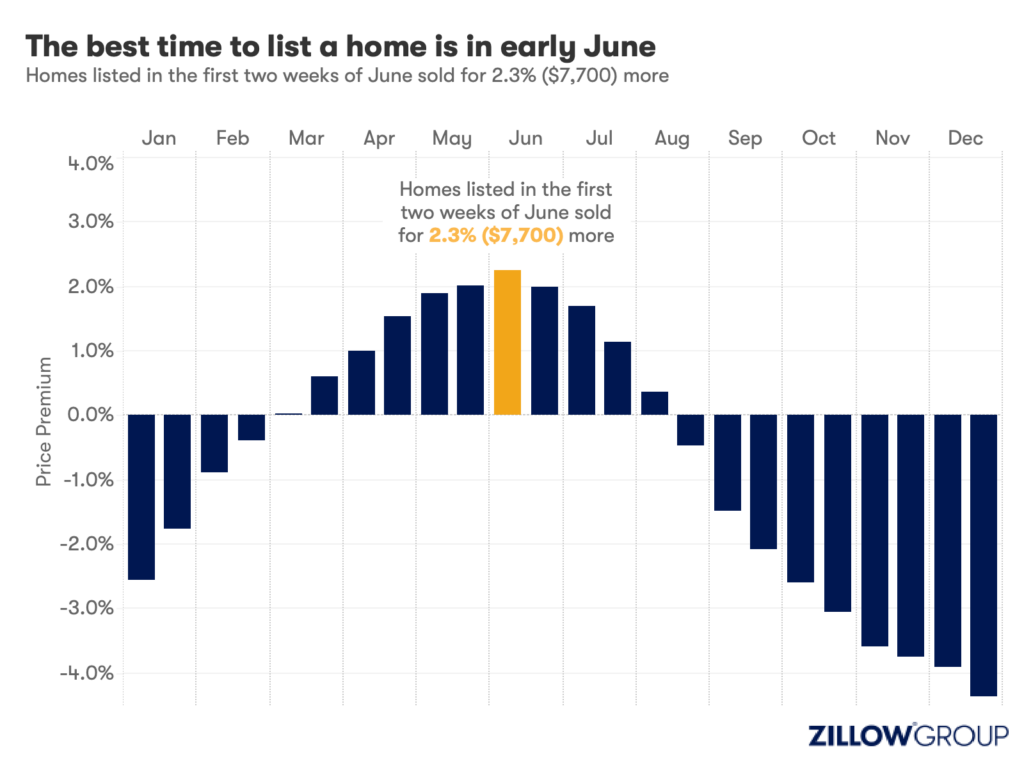
- A Zillow analysis of 2023 home sales finds homes listed in the first two weeks of June sold for 2.3% more.
- The best time to list a home for sale is a month later than it was in 2019, likely driven by mortgage rates.
- The best time to list can be as early as the second half of February in San Francisco, and as late as the first half of July in New York and Philadelphia.
Spring home sellers looking to maximize their sale price may want to wait it out and list their home for sale in the first half of June. A new Zillow® analysis of 2023 sales found that homes listed in the first two weeks of June sold for 2.3% more, a $7,700 boost on a typical U.S. home.
The best time to list consistently had been early May in the years leading up to the pandemic. The shift to June suggests mortgage rates are strongly influencing demand on top of the usual seasonality that brings buyers to the market in the spring. This home-shopping season is poised to follow a similar pattern as that in 2023, with the potential for a second wave if the Federal Reserve lowers interest rates midyear or later.
The 2.3% sale price premium registered last June followed the first spring in more than 15 years with mortgage rates over 6% on a 30-year fixed-rate loan. The high rates put home buyers on the back foot, and as rates continued upward through May, they were still reassessing and less likely to bid boldly. In June, however, rates pulled back a little from 6.79% to 6.67%, which likely presented an opportunity for determined buyers heading into summer. More buyers understood their market position and could afford to transact, boosting competition and sale prices.
The old logic was that sellers could earn a premium by listing in late spring, when search activity hit its peak. Now, with persistently low inventory, mortgage rate fluctuations make their own seasonality. First-time home buyers who are on the edge of qualifying for a home loan may dip in and out of the market, depending on what’s happening with rates. It is almost certain the Federal Reserve will push back any interest-rate cuts to mid-2024 at the earliest. If mortgage rates follow, that could bring another surge of buyers later this year.
Mortgage rates have been impacting affordability and sale prices since they began rising rapidly two years ago. In 2022, sellers nationwide saw the highest sale premium when they listed their home in late March, right before rates barreled past 5% and continued climbing.
Zillow’s research finds the best time to list can vary widely by metropolitan area. In 2023, it was as early as the second half of February in San Francisco, and as late as the first half of July in New York. Thirty of the top 35 largest metro areas saw for-sale listings command the highest sale prices between May and early July last year.
Zillow also found a wide range in the sale price premiums associated with homes listed during those peak periods. At the hottest time of the year in San Jose, homes sold for 5.5% more, a $88,000 boost on a typical home. Meanwhile, homes in San Antonio sold for 1.9% more during that same time period.
| Metropolitan Area | Best Time to List | Price Premium | Dollar Boost |
| United States | First half of June | 2.3% | $7,700 |
| New York, NY | First half of July | 2.4% | $15,500 |
| Los Angeles, CA | First half of May | 4.1% | $39,300 |
| Chicago, IL | First half of June | 2.8% | $8,800 |
| Dallas, TX | First half of June | 2.5% | $9,200 |
| Houston, TX | Second half of April | 2.0% | $6,200 |
| Washington, DC | Second half of June | 2.2% | $12,700 |
| Philadelphia, PA | First half of July | 2.4% | $8,200 |
| Miami, FL | First half of June | 2.3% | $12,900 |
| Atlanta, GA | Second half of June | 2.3% | $8,700 |
| Boston, MA | Second half of May | 3.5% | $23,600 |
| Phoenix, AZ | First half of June | 3.2% | $14,700 |
| San Francisco, CA | Second half of February | 4.2% | $50,300 |
| Riverside, CA | First half of May | 2.7% | $15,600 |
| Detroit, MI | First half of July | 3.3% | $7,900 |
| Seattle, WA | First half of June | 4.3% | $31,500 |
| Minneapolis, MN | Second half of May | 3.7% | $13,400 |
| San Diego, CA | Second half of April | 3.1% | $29,600 |
| Tampa, FL | Second half of June | 2.1% | $8,000 |
| Denver, CO | Second half of May | 2.9% | $16,900 |
| Baltimore, MD | First half of July | 2.2% | $8,200 |
| St. Louis, MO | First half of June | 2.9% | $7,000 |
| Orlando, FL | First half of June | 2.2% | $8,700 |
| Charlotte, NC | Second half of May | 3.0% | $11,000 |
| San Antonio, TX | First half of June | 1.9% | $5,400 |
| Portland, OR | Second half of April | 2.6% | $14,300 |
| Sacramento, CA | First half of June | 3.2% | $17,900 |
| Pittsburgh, PA | Second half of June | 2.3% | $4,700 |
| Cincinnati, OH | Second half of April | 2.7% | $7,500 |
| Austin, TX | Second half of May | 2.8% | $12,600 |
| Las Vegas, NV | First half of June | 3.4% | $14,600 |
| Kansas City, MO | Second half of May | 2.5% | $7,300 |
| Columbus, OH | Second half of June | 3.3% | $10,400 |
| Indianapolis, IN | First half of July | 3.0% | $8,100 |
| Cleveland, OH | First half of July | 3.4% | $7,400 |
| San Jose, CA | First half of June | 5.5% | $88,400 |
The post Homes listed for sale in early June sell for $7,700 more appeared first on Zillow Research.
federal reserve pandemic home sales mortgage rates interest ratesGovernment
Survey Shows Declining Concerns Among Americans About COVID-19
Survey Shows Declining Concerns Among Americans About COVID-19
A new survey reveals that only 20% of Americans view covid-19 as "a major threat"…
A new survey reveals that only 20% of Americans view covid-19 as "a major threat" to the health of the US population - a sharp decline from a high of 67% in July 2020.
What's more, the Pew Research Center survey conducted from Feb. 7 to Feb. 11 showed that just 10% of Americans are concerned that they will catch the disease and require hospitalization.
"This data represents a low ebb of public concern about the virus that reached its height in the summer and fall of 2020, when as many as two-thirds of Americans viewed COVID-19 as a major threat to public health," reads the report, which was published March 7.
According to the survey, half of the participants understand the significance of researchers and healthcare providers in understanding and treating long COVID - however 27% of participants consider this issue less important, while 22% of Americans are unaware of long COVID.
What's more, while Democrats were far more worried than Republicans in the past, that gap has narrowed significantly.
"In the pandemic’s first year, Democrats were routinely about 40 points more likely than Republicans to view the coronavirus as a major threat to the health of the U.S. population. This gap has waned as overall levels of concern have fallen," reads the report.
More via the Epoch Times;
The survey found that three in ten Democrats under 50 have received an updated COVID-19 vaccine, compared with 66 percent of Democrats ages 65 and older.
Moreover, 66 percent of Democrats ages 65 and older have received the updated COVID-19 vaccine, while only 24 percent of Republicans ages 65 and older have done so.
“This 42-point partisan gap is much wider now than at other points since the start of the outbreak. For instance, in August 2021, 93 percent of older Democrats and 78 percent of older Republicans said they had received all the shots needed to be fully vaccinated (a 15-point gap),” it noted.
COVID-19 No Longer an Emergency
The U.S. Centers for Disease Control and Prevention (CDC) recently issued its updated recommendations for the virus, which no longer require people to stay home for five days after testing positive for COVID-19.
The updated guidance recommends that people who contracted a respiratory virus stay home, and they can resume normal activities when their symptoms improve overall and their fever subsides for 24 hours without medication.
“We still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses, this includes vaccination, treatment, and staying home when we get sick,” CDC director Dr. Mandy Cohen said in a statement.
The CDC said that while the virus remains a threat, it is now less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease.
“Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19,” it stated.
The federal government suspended its free at-home COVID-19 test program on March 8, according to a website set up by the government, following a decrease in COVID-19-related hospitalizations.
According to the CDC, hospitalization rates for COVID-19 and influenza diseases remain “elevated” but are decreasing in some parts of the United States.
Government
Rand Paul Teases Senate GOP Leader Run – Musk Says “I Would Support”
Rand Paul Teases Senate GOP Leader Run – Musk Says "I Would Support"
Republican Kentucky Senator Rand Paul on Friday hinted that he may jump…

Republican Kentucky Senator Rand Paul on Friday hinted that he may jump into the race to become the next Senate GOP leader, and Elon Musk was quick to support the idea. Republicans must find a successor for periodically malfunctioning Mitch McConnell, who recently announced he'll step down in November, though intending to keep his Senate seat until his term ends in January 2027, when he'd be within weeks of turning 86.
So far, the announced field consists of two quintessential establishment types: John Cornyn of Texas and John Thune of South Dakota. While John Barrasso's name had been thrown around as one of "The Three Johns" considered top contenders, the Wyoming senator on Tuesday said he'll instead seek the number two slot as party whip.
Paul used X to tease his potential bid for the position which -- if the GOP takes back the upper chamber in November -- could graduate from Minority Leader to Majority Leader. He started by telling his 5.1 million followers he'd had lots of people asking him about his interest in running...
Thousands of people have been asking if I'd run for Senate leadership...
— Rand Paul (@RandPaul) March 8, 2024
...then followed up with a poll in which he predictably annihilated Cornyn and Thune, taking a 96% share as of Friday night, with the other two below 2% each.
????????️VOTE NOW ????️ ???? Who would you like to be the next Senate leader?
— Rand Paul (@RandPaul) March 8, 2024
Elon Musk was quick to back the idea of Paul as GOP leader, while daring Cornyn and Thune to follow Paul's lead by throwing their names out for consideration by the Twitter-verse X-verse.
I would support Rand Paul and suspect that other candidates will not actually run polls out of concern for the results, but let’s see if they will!
— Elon Musk (@elonmusk) March 8, 2024
Paul has been a stalwart opponent of security-state mass surveillance, foreign interventionism -- to include shoveling billions of dollars into the proxy war in Ukraine -- and out-of-control spending in general. He demonstrated the latter passion on the Senate floor this week as he ridiculed the latest kick-the-can spending package:
This bill is an insult to the American people. The earmarks are all the wasteful spending that you could ever hope to see, and it should be defeated. Read more: https://t.co/Jt8K5iucA4 pic.twitter.com/I5okd4QgDg
— Senator Rand Paul (@SenRandPaul) March 8, 2024
In February, Paul used Senate rules to force his colleagues into a grueling Super Bowl weekend of votes, as he worked to derail a $95 billion foreign aid bill. "I think we should stay here as long as it takes,” said Paul. “If it takes a week or a month, I’ll force them to stay here to discuss why they think the border of Ukraine is more important than the US border.”
Don't expect a Majority Leader Paul to ditch the filibuster -- he's been a hardy user of the legislative delay tactic. In 2013, he spoke for 13 hours to fight the nomination of John Brennan as CIA director. In 2015, he orated for 10-and-a-half-hours to oppose extension of the Patriot Act.
Among the general public, Paul is probably best known as Capitol Hill's chief tormentor of Dr. Anthony Fauci, who was director of the National Institute of Allergy and Infectious Disease during the Covid-19 pandemic. Paul says the evidence indicates the virus emerged from China's Wuhan Institute of Virology. He's accused Fauci and other members of the US government public health apparatus of evading questions about their funding of the Chinese lab's "gain of function" research, which takes natural viruses and morphs them into something more dangerous. Paul has pointedly said that Fauci committed perjury in congressional hearings and that he belongs in jail "without question."
Musk is neither the only nor the first noteworthy figure to back Paul for party leader. Just hours after McConnell announced his upcoming step-down from leadership, independent 2024 presidential candidate Robert F. Kennedy, Jr voiced his support:
Mitch McConnell, who has served in the Senate for almost 40 years, announced he'll step down this November.
— Robert F. Kennedy Jr (@RobertKennedyJr) February 28, 2024
Part of public service is about knowing when to usher in a new generation. It’s time to promote leaders in Washington, DC who won’t kowtow to the military contractors or…
In a testament to the extent to which the establishment recoils at the libertarian-minded Paul, mainstream media outlets -- which have been quick to report on other developments in the majority leader race -- pretended not to notice that Paul had signaled his interest in the job. More than 24 hours after Paul's test-the-waters tweet-fest began, not a single major outlet had brought it to the attention of their audience.
That may be his strongest endorsement yet.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 International3 days ago
International3 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex





















