Uncategorized
Nonviral Platforms Streamline Gene Therapy Delivery
Complications of viral vectors can be avoided with DNA plasmids, engineered vesicles, cell-penetrating proteins, and other nonviral alternatives. To skirt…

By Ashleen Knutsen
There is perhaps no better example of getting to the root of a problem than gene therapy. This medical treatment alters the very building blocks of life, and it has the potential to treat or cure not just genetic diseases, but cancers and infectious diseases as well.
For years, scientists have been focusing on whether or not these treatments can work. Now armed with evidence in the affirmative, they have been devoting more of their attention to exploring the safest and most efficient ways of introducing these treatments to host cells.
For these types of treatments, viruses seem like the obvious choice, as they are designed to infiltrate cells. As such, all FDA-approved gene therapies are delivered using viral vectors, including viral vectors based on adeno-associated virus (AAV), lentivirus, or herpes simplex virus. However, while viral vectors have met with some success, they have also raised doubts. Potential risks of viral vectors include liver toxicity, immunogenicity, and oncogenicity. In addition, viral vectors have a relatively small packaging capacity, and they’re difficult to produce at scale because they rely on costly and time-
consuming manufacturing processes.
To skirt the limitations associated with viral vectors, scientists are turning to nonviral delivery systems. However, unlike viruses, nonviral delivery systems lack the natural ability to enter cells. Instead, nonviral delivery systems must rely on physical or chemical mechanisms.
Transforming the eye into a biofactory
Because the eye is accessible and immune privileged, it is a prime target for physical delivery approaches such as the transfer of genetic material via electroporation, which uses an electrical pulse to create temporary pores in cell membranes. With electroporation, genetic material such as DNA plasmids can overcome natural barriers of the eye that often thwart viral vectors. Also, DNA plasmids can target specific types of retinal cells.
“Viral vectors, when delivered into the vitreous, may not effectively target specific retinal cell types or regions, leading to nonspecific gene expression,” observes Thierry Bordet, PhD, chief scientific officer at Eyevensys. “This lack of cell-type specificity can result in off-target effects or inadequate transduction of the desired retinal cells, thereby reducing the effectiveness of the treatment.”
Instead of viral vectors, Eyevensys uses DNA plasmids encoding therapeutic proteins to treat a variety of retinal diseases, such as wet age-related macular degeneration and geographic atrophy.
Compared with viral vectors, DNA plasmids are less immunogenic, allowing for redosing, and they don’t integrate into the genome, eliminating concerns of mutagenesis. DNA plasmids can also accommodate larger DNA sequences and are relatively easy to manufacture, purify, and produce at scale.
Because DNA plasmids have a lower transfection efficiency than viral vectors, Eyevensys applies electroporation. Specifically, the company uses a proprietary ocular device and pulse generator to help transgenes enter ciliary muscle cells. Within the cells, the transgenes are translated into therapeutic proteins that can reach all parts of the eye. Six months later, the proteins are still found, and side effects are not detected.
A bigger and better biomaterial
Besides physical mechanisms, there are chemical approaches to delivering gene therapy. Chemical approaches that use materials compatible with the human body are less likely to generate immune responses. However, some popular choices, such as lipid nanoparticles, can trigger the immune response as they carry a therapeutic cargo into the cytoplasm.
To address this limitation, Vesigen Therapeutics produces engineered versions of naturally occurring extracellular vesicles called ARMMs (ARRDC1-mediated microvesicles). In the engineered ARMMs, the ARRDC1 protein is attached via a linker to a payload molecule. A variety of payloads may be carried across the cytoplasm without causing immunogenicity.

ARMMs have a large payload capacity—large enough to deliver base editing complexes, which are too large for most delivery platforms. Some companies have resorted to splitting base editors so that they may be delivered via separate AAVs and pieced together inside the cell. This significantly increases complexity and cost. ARMMs would likely overcome these challenges, allowing for the delivery of multiple gene editors and gRNA complexes in their fully functional form within a single particle. In addition, unlike viral vectors, ARMMs are transient.
“When you deliver a genome editor, you don’t want it to persist in the cell,” says Joseph Nabhan, PhD, chief scientific officer at Vesigen. “You want that genome editor to be in the cell and the nucleus for a short period of time to edit the target gene and then get degraded. And that’s what virally delivered therapeutics cannot do.”
In vitro tests have demonstrated efficient editing of multiple genomic loci across a range of human and murine cell types and robust modification of the target gene product. Additionally, an in vivo murine study found that a single intranasal administration of ARMMs loaded with gene or base editors targeting immune modulatory genes resulted in 60% or greater on-target editing in macrophages, blunting the release of associated cytokines in lung tissue.
Defying all odds
Another company taking inspiration from a natural mechanism to create a nonviral delivery system is CyGenica. In CyGenica’s case, the natural mechanism is the kind of cell penetration that is accomplished by certain proteins. Such proteins typically have a positive charge and exploit endocytosis. CyGenica, however, has engineered a cell-penetrating protein that has a negative charge and eschews endocytosis.
“When you have a positively charged molecule interacting with a negatively charged cell membrane, it binds strongly and destroys the membrane,” says Nusrat Sanghamitra, PhD, CEO, CSO, and founder of CyGenica. “With our platform, that doesn’t happen because we use a negatively charged molecule.”

This molecule works like a molecular drill. According to CyGenica, it “deterministically translocates through the cell membrane using a non-endocytic, helical mechanism.”
CyGenica has named its platform GEENIE, which stands for Guided Efficient, and Effortless Navigation. Unlike other delivery platforms, GEENIE directly interacts with the cell membrane and goes through it without making any permanent hole or causing any damage. GEENIE lets the membrane reseal without forming endosomes, which entrap up to 98% of therapeutic molecules.
“Due to the direct cell entry mechanism, GEENIE bypasses the endosomes,” Sanghamitra asserts. “And as opposed to just 2 to 3%, 100% of our platform is available to reach the intracellular target site.”
According to CyGenica, GEENIE is not only effective but also very safe. The company reports that GEENIE has been used to deliver CRISPR gene editing systems into cells without causing any toxicity, and that a high dose (1,800 mg/kg) of the platform in mice resulted in no signs of toxicity or immunogenicity.
CyGenica is currently developing its platform to deliver small-molecule chemotherapy drugs. The company claims that GEENIE can work with any cargo, including RNA molecules, and that it can be made to target any cell type with a known binding partner. Finally, the company says that in vivo studies in mice have demonstrated a good biodistribution of GEENIE throughout the body, including the brain.
Producing a better cell
Ensuring that cell health is maintained is an essential step in the development of therapeutics like gene therapy. One company that takes cell health especially seriously is Avectas, a specialist in the ex vivo delivery of molecules such as mRNA, proteins, and gene editing payloads for cell engineering applications.
“We’re focused on providing a technology for therapeutic developers which produces a gene edited cell with superior health and function compared with existing technologies, creating a more efficacious product,” says Justin McCue, PhD, chief technology officer at Avectas.
The company has developed an ex vivo cell editing technology called Solupore. Inside a closed single-use transfection chamber, cells are engineered by spraying them with a novel solution containing a gene editing cargo as well as an atomizer that creates a transient permeabilization to the cell membrane.

This process takes approximately 10 minutes—a small fraction of a typical cell manufacturing duration. After the process is complete, edited cells are expanded and tests are performed to ensure that quality control and regulatory goals are met. Cells that pass the tests may be introduced to patients.
So far, Solupore has been tested with T cells, natural killer cells, and induced pluripotent stem cells. According to Avectas, in vitro analytical tests—including assays for cell viability, phenotype, apoptosis, avidity, and cytoxicity—have shown that T cells edited using Solupore perform better than cells modified by other methods. The company adds that it used a mouse tumor model to show that in vivo Solupore-edited T cells engrafted better and were more cytotoxic than cells that were produced using existing cell editing technology.
“With the next generation of cell therapy products, we’re seeing an evolution in the industry to produce healthy and more highly functioning cells, potentially reducing the dose requirements based on improved efficacy,” McCue says. “For next-generation cell therapies that require gene modification, Solupore provides the potential to meet that goal.”
The post Nonviral Platforms Streamline Gene Therapy Delivery appeared first on GEN - Genetic Engineering and Biotechnology News.
treatment fda genome genetic therapy rna dnaUncategorized
One city held a mass passport-getting event
A New Orleans congressman organized a way for people to apply for their passports en masse.

While the number of Americans who do not have a passport has dropped steadily from more than 80% in 1990 to just over 50% now, a lack of knowledge around passport requirements still keeps a significant portion of the population away from international travel.
Over the four years that passed since the start of covid-19, passport offices have also been dealing with significant backlog due to the high numbers of people who were looking to get a passport post-pandemic.
Related: Here is why it is (still) taking forever to get a passport
To deal with these concurrent issues, the U.S. State Department recently held a mass passport-getting event in the city of New Orleans. Called the "Passport Acceptance Event," the gathering was held at a local auditorium and invited residents of Louisiana’s 2nd Congressional District to complete a passport application on-site with the help of staff and government workers.
'Come apply for your passport, no appointment is required'
"Hey #LA02," Rep. Troy A. Carter Sr. (D-LA), whose office co-hosted the event alongside the city of New Orleans, wrote to his followers on Instagram (META) . "My office is providing passport services at our #PassportAcceptance event. Come apply for your passport, no appointment is required."
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
The event was held on March 14 from 10 a.m. to 1 p.m. While it was designed for those who are already eligible for U.S. citizenship rather than as a way to help non-citizens with immigration questions, it helped those completing the application for the first time fill out forms and make sure they have the photographs and identity documents they need. The passport offices in New Orleans where one would normally have to bring already-completed forms have also been dealing with lines and would require one to book spots weeks in advance.
These are the countries with the highest-ranking passports in 2024
According to Carter Sr.'s communications team, those who submitted their passport application at the event also received expedited processing of two to three weeks (according to the State Department's website, times for regular processing are currently six to eight weeks).
While Carter Sr.'s office has not released the numbers of people who applied for a passport on March 14, photos from the event show that many took advantage of the opportunity to apply for a passport in a group setting and get expedited processing.
Every couple of months, a new ranking agency puts together a list of the most and least powerful passports in the world based on factors such as visa-free travel and opportunities for cross-border business.
In January, global citizenship and financial advisory firm Arton Capital identified United Arab Emirates as having the most powerful passport in 2024. While the United States topped the list of one such ranking in 2014, worsening relations with a number of countries as well as stricter immigration rules even as other countries have taken strides to create opportunities for investors and digital nomads caused the American passport to slip in recent years.
A UAE passport grants holders visa-free or visa-on-arrival access to 180 of the world’s 198 countries (this calculation includes disputed territories such as Kosovo and Western Sahara) while Americans currently have the same access to 151 countries.
stocks pandemic covid-19 grantsUncategorized
Fast-food chain closes restaurants after Chapter 11 bankruptcy
Several major fast-food chains recently have struggled to keep restaurants open.

Competition in the fast-food space has been brutal as operators deal with inflation, consumers who are worried about the economy and their jobs and, in recent months, the falling cost of eating at home.
Add in that many fast-food chains took on more debt during the covid pandemic and that labor costs are rising, and you have a perfect storm of problems.
It's a situation where Restaurant Brands International (QSR) has suffered as much as any company.
Related: Wendy's menu drops a fan favorite item, adds something new
Three major Burger King franchise operators filed for bankruptcy in 2023, and the chain saw hundreds of stores close. It also saw multiple Popeyes franchisees move into bankruptcy, with dozens of locations closing.
RBI also stepped in and purchased one of its key franchisees.
"Carrols is the largest Burger King franchisee in the United States today, operating 1,022 Burger King restaurants in 23 states that generated approximately $1.8 billion of system sales during the 12 months ended Sept. 30, 2023," RBI said in a news release. Carrols also owns and operates 60 Popeyes restaurants in six states."
The multichain company made the move after two of its large franchisees, Premier Kings and Meridian, saw multiple locations not purchased when they reached auction after Chapter 11 bankruptcy filings. In that case, RBI bought select locations but allowed others to close.
Image source: Chen Jianli/Xinhua via Getty
Another fast-food chain faces bankruptcy problems
Bojangles may not be as big a name as Burger King or Popeye's, but it's a popular chain with more than 800 restaurants in eight states.
"Bojangles is a Carolina-born restaurant chain specializing in craveable Southern chicken, biscuits and tea made fresh daily from real recipes, and with a friendly smile," the chain says on its website. "Founded in 1977 as a single location in Charlotte, our beloved brand continues to grow nationwide."
Like RBI, Bojangles uses a franchise model, which makes it dependent on the financial health of its operators. The company ultimately saw all its Maryland locations close due to the financial situation of one of its franchisees.
Unlike. RBI, Bojangles is not public — it was taken private by Durational Capital Management LP and Jordan Co. in 2018 — which means the company does not disclose its financial information to the public.
That makes it hard to know whether overall softness for the brand contributed to the chain seeing its five Maryland locations after a Chapter 11 bankruptcy filing.
Bojangles has a messy bankruptcy situation
Even though the locations still appear on the Bojangles website, they have been shuttered since late 2023. The locations were operated by Salim Kakakhail and Yavir Akbar Durranni. The partners operated under a variety of LLCs, including ABS Network, according to local news channel WUSA9.
The station reported that the owners face a state investigation over complaints of wage theft and fraudulent W2s. In November Durranni and ABS Network filed for bankruptcy in New Jersey, WUSA9 reported.
"Not only do former employees say these men owe them money, WUSA9 learned the former owners owe the state, too, and have over $69,000 in back property taxes."
Former employees also say that the restaurant would regularly purchase fried chicken from Popeyes and Safeway when it ran out in their stores, the station reported.
Bojangles sent the station a comment on the situation.
"The franchisee is no longer in the Bojangles system," the company said. "However, it is important to note in your coverage that franchisees are independent business owners who are licensed to operate a brand but have autonomy over many aspects of their business, including hiring employees and payroll responsibilities."
Kakakhail and Durranni did not respond to multiple requests for comment from WUSA9.
bankruptcy pandemicUncategorized
Industrial Production Increased 0.1% in February
From the Fed: Industrial Production and Capacity Utilization
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 p…
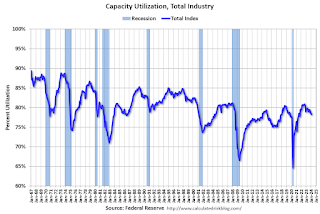
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 percent. Both gains partly reflected recoveries from weather-related declines in January. The index for utilities fell 7.5 percent in February because of warmer-than-typical temperatures. At 102.3 percent of its 2017 average, total industrial production in February was 0.2 percent below its year-earlier level. Capacity utilization for the industrial sector remained at 78.3 percent in February, a rate that is 1.3 percentage points below its long-run (1972–2023) average.Click on graph for larger image.
emphasis added
This graph shows Capacity Utilization. This series is up from the record low set in April 2020, and above the level in February 2020 (pre-pandemic).
Capacity utilization at 78.3% is 1.3% below the average from 1972 to 2022. This was below consensus expectations.
Note: y-axis doesn't start at zero to better show the change.
 The second graph shows industrial production since 1967.
The second graph shows industrial production since 1967.Industrial production increased to 102.3. This is above the pre-pandemic level.
Industrial production was above consensus expectations.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International1 week ago
International1 week agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 week ago
International1 week agoWalmart launches clever answer to Target’s new membership program
-

 Spread & Containment3 days ago
Spread & Containment3 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex



















