Uncategorized
Liquid Biopsy Technologies Hasten Progress in Precision Oncology
As more companies invest in developing and refining their liquid biopsy technologies and entering key partnerships, it is likely that there are even more…

By Kathy Liszewski
For those with cancer, precision oncology promises better treatment plans. It is about allowing treatments to be selected on the basis of genetic signatures, which can reveal specific tumorigenic mechanisms. Crucially, when these mechanisms are identified, it is possible to select targeted treatments. This approach works best when the genetic signatures underlying tumorigenesis are seen most clearly. Fortunately, the eyes on tumorigenesis include the liquid biopsy. It involves the scrutiny of biological fluids, such as blood or urine, for early cancer detection, stratification, and personalized treatment.
Recently, the 7th Annual Liquid Biopsy for Precision Oncology Summit highlighted advances and challenges in the field. Advances include systems to profile circulating tumor DNA (ctDNA), as well as systems to evaluate tumor mutational burden (TMB). Challenges include harnessing artificial intelligence to create more multidimensional views of patients’ cancers. Another challenge is applying recent research. Exosome research, for example, has led to a urine-based liquid biopsy for stratifying prostate cancer patients.
Comprehensive genomic profiling
Like a fingerprint left at a crime scene, ctDNA implicates a perpetrator—in this case, a DNA-shedding tumor. Performing a liquid biopsy by analyzing a cancer patient’s blood sample can reveal not only the specific type of tumor but also whether the tumor is likely to respond to an available treatment.
Foundation Medicine
Foundation Medicine manufactures and provides diagnostic testing that helps physicians understand a cancer patient’s DNA alterations. Lucas Dennis, PhD, the company’s senior vice president and head of R&D, explains, “One of the challenges for liquid biopsies is the need for accurate mirroring of cancer tissue samples. Liquid biopsies—for example, in blood—must provide very sensitive testing of even rare alterations in tumor tissue. Our company develops high-quality, robust, and very specific tests to identify in blood the same alterations found in tissue testing. We also serve as a centralized testing laboratory.”
The company’s FoundationOne Liquid CDx liquid biopsy focuses on four major genetic alterations: short variants, insertions and deletions, rearrangements (including fusions), and copy number effects. Dennis elaborates, “We separate the plasma from blood and then extract nucleic acid. Next, we perform DNA sequencing, providing targeted gene results across 324 cancer-relevant genes. The results are often clinically actionable. Our reports are specifically annotated [with information from] scientific articles, drug reports, and other materials.”
According to Dennis, Foundation Medicine’s platform is also being utilized for developing and analyzing applicability to companion diagnostics, that is, assessing whether a patient will respond to a specific drug therapy. He asserts that the company has “a number of companion diagnostics approved on the platform.”
Looking to the future, Dennis observes, “Liquid biopsy is not just about comprehensive genomic profiling or the identification of alterations to inform therapy selection. It is also becoming an important tool for monitoring treatment response. We are working on different applications—for example, to evaluate serial ctDNA testing that will gather
early as well as long-term treatment
response insights.”
Tumor mutational burden as a cancer biomarker
Blood-based liquid biopsies are also being studied for assessment of TMB. “TMB is the number of somatic mutations detected in a tumor and is a surrogate for neoantigen load,” explains Sarah Shagan, PhD, principal scientific manager, biomarker lead, Genentech, a member of the Roche Group. Earlier studies demonstrated that higher TMB in tissue was associated with better outcomes from immuno-oncology therapy.

Genentech
“While numerous cancer types are characterized by higher mean TMBs, the original experiments were done in non-small-cell lung cancer,” Shagan continues. “Thus, lung cancer has been one of the leading indications for the development of TMB as a biomarker.”
Shagan reports that Genentech scientists co-developed and validated an assay with Foundation Medicine that eventually became part of the FoundationOne Liquid CDx liquid biopsy. “The concept of blood TMB (bTMB),” she notes, “was to see if detection of TMB in the blood could select patients most likely to benefit from immune-
oncology therapy without having to use a tissue biopsy, which is not always available or always sufficient for next-generation sequencing in metastatic non-small-cell lung cancer patients.”
Genentech initiated a Phase III study (BFAST Cohort C) comparing chemotherapy versus treatment with atezolizumab in patients with high bTMB. Atezolizu-mab is an FDA-approved non-small-cell lung cancer therapeutic, a monoclonal anti-PD-L1 antibody that enhances
tumor-specific T-cell responses. The primary endpoint of the Phase III study was measurement of progression-free survival. The group saw a trend toward improved progression-free survival using the blood liquid biopsy assay.
According to Shagan, the assay technology is still young and subject to improvement. “Perhaps improvements in how we measure bTMB that are ongoing across the industry could lead to a more robust biomarker,” she suggests. “Combination approaches with other blood-based biomarkers could also be an avenue to capturing additional biology that could predict outcomes to immunotherapy. It’s still early days, but the potential uses for ctDNA and the signals we have seen so far make it an exciting field that may provide great benefits to patients.”

A multidimensional approach
Although many scientists believe that the epigenome can potentially reveal 10 times more information about cancer and what drives it than the genome does, those attempting to access this information, especially via liquid biopsy, have faced several difficulties, including a lack of robust methodologies.

Guardant Health
To overcome some of these difficulties and capture epigenomic insights in a practical way, Guardant Health recently expanded its collection of liquid biopsy platforms by introducing the Guardant Infinity platform. It is, according to Shile Zhang, PhD, associate director of bioinformatics at Guardant, the “first commercially available blood-based technology to combine epigenomic and genomic profiling with artificial intelligence to offer a multidimensional view of a patient’s cancer.”
How does it work? Zhang explains, “The technology uses a novel nondestructive technique for the enrichment of methylated DNA molecules, which has proven to be critical for highly efficient, sensitive, and scalable sequencing. Improving the noncancer background depletion step further improves the signal-to-noise ratio of tumor detection and enables simultaneous capture of a large footprint of the genome. The high molecule recovery rate of the technology lowers DNA sample input requirements, facilitating compatibility with plasma samples isolated from a single blood draw—samples that typically have low DNA yields.”
The company employs its new technology for drug discovery and development research as well as multiple clinical applications. “We are already using the technology for biomarker discovery associated with virtually any type of solid cancer,” Zhang relates. “In commercially launched clinical applications, we are using the technology for ‘minimal residual disease’ detection in colorectal, breast, and lung cancers and for screening in colorectal cancer. We plan to make multidimensional liquid biopsy technology available soon for tumor characterization and quantification for all solid tumors.”
As for the future of this evolving platform, Zhang projects, “The potential goes well beyond cancer. Almost every disease imaginable has a robust fingerprint in the epigenomic domain. These insights can shape our understanding of a myriad of conditions ranging from heart disease to neurodegenerative diseases.”
Combining liquid biopsy with tissue analysis
Despite its many advantages, contemporary liquid biopsies also suffer from important limitations. “Sensitivity can be limited at low disease burden and by variability in tumor DNA shedding,” reports Nike Beaubier, MD, senior vice president, Tempus Labs. “Additionally, accuracy of somatic mutation calling is limited by the inclusion of germline mutations and clonal hematopoiesis of indeterminate potential (CHIP).” (CHIP, which is particularly common in older people, occurs when hematopoietic stem cells or other cell progenitors give rise to clones that share unique mutations.)

Tempus Labs
Beaubier says that in liquid biopsies, limitations in sensitivity and accuracy can be largely compensated for by tumor/normal matched tissue sequencing, which generates data that can be used to accurately distinguish somatic and germline alterations. “If the source of normal DNA is blood, this data can also be used to subtract CHIP,” she advises. “Likewise, the limitations of tissue sequencing—the necessity for archival tissue or a procedure, and the inability to assess heterogeneity across tumor sites—can be compensated for with a liquid biopsy.
“We favor a combined approach with concurrent tissue and plasma testing at diagnosis. Physicians also apply our liquid biopsies for ongoing patient monitoring throughout treatment, which provides critical longitudinal data that can inform future treatment.”
The company provides two liquid biopsy assays, the xF and the xF+. Beaubier elaborates, “The xF is a 105-gene panel, covering genes of major clinical importance including resistance genes. Our more comprehensive assay, the xF+, is a 523-gene panel. Single nucleotide variants and insertions/deletions are called in all genes, and translocations/gene rearrangements and copy number changes are detected in a clinically important subset of genes.”
Both tests can determine whether samples can be classified as microsatellite instability-high, and xF+ has enough genomic space to measure tumor mutational burden, which may serve as a biomarker to predict how patients will respond to various cancer immunotherapies. “Crucially for longitudinal monitoring, both assays also have a circulating tumor fraction calculation,” Beaubier adds.
Although liquid biopsy continues to evolve, challenges remain that must be addressed. Beaubier reflects, “One enhancement would be to assay the buffy coat in parallel with plasma to get immune repertoire data, which could be used to assess immunotherapy options in more detail and to monitor response. The addition of other analytes, including protein tumor markers, could increase sensitivity. More robust clinical studies are needed to show that liquid biopsy tumor burden trends, such as circulating tumor fraction, can accurately predict imaging results and change the balance of follow-up from radiology to molecular assays.”
Exosomes as cancer diagnostics
Exosome research has exploded in the last decade. Exosomes, the tiny vesicles released by cells, contain bioactive molecules such as nucleic acids, proteins, and lipids that can reflect altered physiological and pathological states of their parental cells. Further, because they are found in abundance in body fluids, exosomes are being exploited for clinical applications, including liquid biopsy.
“The field of exosome-based liquid biopsy has grown quickly over the last 15 years,” states Johan Skog, PhD, a founding scientist and the chief scientific officer at Exosome Diagnostics, a Bio-Techne brand. A decade and a half ago, Skog coauthored a seminal paper demonstrating that tumor-derived mutations could be found in plasma exosomal RNA (Skog et al. Nat Cell Biol. 2008; 10: 1470–1476). “In that same year,” he says, “Exosome Diagnostics was founded to commercialize the findings, and it later became part of the Bio-Techne brand.”

The company offers a liquid biopsy it calls ExoDx Prostate, which evaluates exosomes in patient urine samples. “The test,” Skog points out, “is included in the National Comprehensive Cancer Network guidelines for early prostate cancer detection.” As the most common type of cancer in men, prostate cancer accounts for more than a quarter of new cancer cases. Alongside digital rectal exams or evaluation of prostate-specific antigen levels, exosomal evaluation via the ExoDx test can stratify patients from low- to high-grade prostate cancer. It is especially useful when prostate-specific antigen results fall into the “gray zone” for cancer risk.
Skog says the company’s ExoLution products can isolate exosomes from biofluids such as plasma, urine, cerebrospinal fluid, and saliva. “They can also co-isolate all the cell-free DNA in a single step from the same sample, maximizing the information obtained from a clinical sample,” he continues. “Co-isolation of exosome RNA and cell-free DNA maximizes the sensitivity for detecting rare tumor-derived mutations and serves as an example of how our platform improves upon analyzing cell-free DNA alone. Multiomic readouts provided by the exosomal platform using the same sample allows for maximization of information obtained from precious clinical samples, both for diagnostic applications and pharmaceutical clinical trials.”
Going forward
As more companies invest in developing and refining their liquid biopsy technologies and entering key partnerships, it is likely that there are even more innovations on the horizon that will address key challenges of sensitivity, precision, and accessibility.
The post Liquid Biopsy Technologies Hasten Progress in Precision Oncology appeared first on GEN - Genetic Engineering and Biotechnology News.
treatment testing fda clinical trials genome genetic therapy rna dna recoveryUncategorized
One city held a mass passport-getting event
A New Orleans congressman organized a way for people to apply for their passports en masse.

While the number of Americans who do not have a passport has dropped steadily from more than 80% in 1990 to just over 50% now, a lack of knowledge around passport requirements still keeps a significant portion of the population away from international travel.
Over the four years that passed since the start of covid-19, passport offices have also been dealing with significant backlog due to the high numbers of people who were looking to get a passport post-pandemic.
Related: Here is why it is (still) taking forever to get a passport
To deal with these concurrent issues, the U.S. State Department recently held a mass passport-getting event in the city of New Orleans. Called the "Passport Acceptance Event," the gathering was held at a local auditorium and invited residents of Louisiana’s 2nd Congressional District to complete a passport application on-site with the help of staff and government workers.
'Come apply for your passport, no appointment is required'
"Hey #LA02," Rep. Troy A. Carter Sr. (D-LA), whose office co-hosted the event alongside the city of New Orleans, wrote to his followers on Instagram (META) . "My office is providing passport services at our #PassportAcceptance event. Come apply for your passport, no appointment is required."
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
The event was held on March 14 from 10 a.m. to 1 p.m. While it was designed for those who are already eligible for U.S. citizenship rather than as a way to help non-citizens with immigration questions, it helped those completing the application for the first time fill out forms and make sure they have the photographs and identity documents they need. The passport offices in New Orleans where one would normally have to bring already-completed forms have also been dealing with lines and would require one to book spots weeks in advance.
These are the countries with the highest-ranking passports in 2024
According to Carter Sr.'s communications team, those who submitted their passport application at the event also received expedited processing of two to three weeks (according to the State Department's website, times for regular processing are currently six to eight weeks).
While Carter Sr.'s office has not released the numbers of people who applied for a passport on March 14, photos from the event show that many took advantage of the opportunity to apply for a passport in a group setting and get expedited processing.
Every couple of months, a new ranking agency puts together a list of the most and least powerful passports in the world based on factors such as visa-free travel and opportunities for cross-border business.
In January, global citizenship and financial advisory firm Arton Capital identified United Arab Emirates as having the most powerful passport in 2024. While the United States topped the list of one such ranking in 2014, worsening relations with a number of countries as well as stricter immigration rules even as other countries have taken strides to create opportunities for investors and digital nomads caused the American passport to slip in recent years.
A UAE passport grants holders visa-free or visa-on-arrival access to 180 of the world’s 198 countries (this calculation includes disputed territories such as Kosovo and Western Sahara) while Americans currently have the same access to 151 countries.
stocks pandemic covid-19 grantsUncategorized
Fast-food chain closes restaurants after Chapter 11 bankruptcy
Several major fast-food chains recently have struggled to keep restaurants open.

Competition in the fast-food space has been brutal as operators deal with inflation, consumers who are worried about the economy and their jobs and, in recent months, the falling cost of eating at home.
Add in that many fast-food chains took on more debt during the covid pandemic and that labor costs are rising, and you have a perfect storm of problems.
It's a situation where Restaurant Brands International (QSR) has suffered as much as any company.
Related: Wendy's menu drops a fan favorite item, adds something new
Three major Burger King franchise operators filed for bankruptcy in 2023, and the chain saw hundreds of stores close. It also saw multiple Popeyes franchisees move into bankruptcy, with dozens of locations closing.
RBI also stepped in and purchased one of its key franchisees.
"Carrols is the largest Burger King franchisee in the United States today, operating 1,022 Burger King restaurants in 23 states that generated approximately $1.8 billion of system sales during the 12 months ended Sept. 30, 2023," RBI said in a news release. Carrols also owns and operates 60 Popeyes restaurants in six states."
The multichain company made the move after two of its large franchisees, Premier Kings and Meridian, saw multiple locations not purchased when they reached auction after Chapter 11 bankruptcy filings. In that case, RBI bought select locations but allowed others to close.
Image source: Chen Jianli/Xinhua via Getty
Another fast-food chain faces bankruptcy problems
Bojangles may not be as big a name as Burger King or Popeye's, but it's a popular chain with more than 800 restaurants in eight states.
"Bojangles is a Carolina-born restaurant chain specializing in craveable Southern chicken, biscuits and tea made fresh daily from real recipes, and with a friendly smile," the chain says on its website. "Founded in 1977 as a single location in Charlotte, our beloved brand continues to grow nationwide."
Like RBI, Bojangles uses a franchise model, which makes it dependent on the financial health of its operators. The company ultimately saw all its Maryland locations close due to the financial situation of one of its franchisees.
Unlike. RBI, Bojangles is not public — it was taken private by Durational Capital Management LP and Jordan Co. in 2018 — which means the company does not disclose its financial information to the public.
That makes it hard to know whether overall softness for the brand contributed to the chain seeing its five Maryland locations after a Chapter 11 bankruptcy filing.
Bojangles has a messy bankruptcy situation
Even though the locations still appear on the Bojangles website, they have been shuttered since late 2023. The locations were operated by Salim Kakakhail and Yavir Akbar Durranni. The partners operated under a variety of LLCs, including ABS Network, according to local news channel WUSA9.
The station reported that the owners face a state investigation over complaints of wage theft and fraudulent W2s. In November Durranni and ABS Network filed for bankruptcy in New Jersey, WUSA9 reported.
"Not only do former employees say these men owe them money, WUSA9 learned the former owners owe the state, too, and have over $69,000 in back property taxes."
Former employees also say that the restaurant would regularly purchase fried chicken from Popeyes and Safeway when it ran out in their stores, the station reported.
Bojangles sent the station a comment on the situation.
"The franchisee is no longer in the Bojangles system," the company said. "However, it is important to note in your coverage that franchisees are independent business owners who are licensed to operate a brand but have autonomy over many aspects of their business, including hiring employees and payroll responsibilities."
Kakakhail and Durranni did not respond to multiple requests for comment from WUSA9.
bankruptcy pandemicUncategorized
Industrial Production Increased 0.1% in February
From the Fed: Industrial Production and Capacity Utilization
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 p…
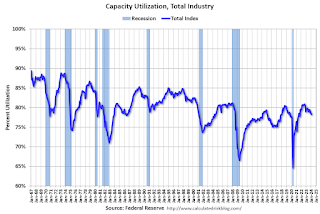
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 percent. Both gains partly reflected recoveries from weather-related declines in January. The index for utilities fell 7.5 percent in February because of warmer-than-typical temperatures. At 102.3 percent of its 2017 average, total industrial production in February was 0.2 percent below its year-earlier level. Capacity utilization for the industrial sector remained at 78.3 percent in February, a rate that is 1.3 percentage points below its long-run (1972–2023) average.Click on graph for larger image.
emphasis added
This graph shows Capacity Utilization. This series is up from the record low set in April 2020, and above the level in February 2020 (pre-pandemic).
Capacity utilization at 78.3% is 1.3% below the average from 1972 to 2022. This was below consensus expectations.
Note: y-axis doesn't start at zero to better show the change.
 The second graph shows industrial production since 1967.
The second graph shows industrial production since 1967.Industrial production increased to 102.3. This is above the pre-pandemic level.
Industrial production was above consensus expectations.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International1 week ago
International1 week agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 week ago
International1 week agoWalmart launches clever answer to Target’s new membership program
-

 Spread & Containment2 days ago
Spread & Containment2 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex



















