Extracellular Signaling Pathway Inhibits SARS-CoV-2 mRNA Expression
One of the outstanding mysteries of COVID-19 is how the SARS-CoV-2 virus can cause so much damage throughout the body — heart, liver, brain, kidneys,…
One of the outstanding mysteries of COVID-19 is how the SARS-CoV-2 virus can cause so much damage throughout the body — heart, liver, brain, kidneys, and the list goes on — without necessarily infecting all of those tissues. A recent study by Abhijit Basu et al. has given us new insight into how, at least some of this damage, might occur. The key discovery is that the SARS-CoV-2 virus need not enter a cell to disturb its function in profound ways; it may actually be sufficient for the spike (S) protein to bind to the outside of a cell to cause significant, and even lethal, changes. Although the puzzle is far from complete, their work shines some light into a dark corner of COVID-19 pathogenesis.
Background: Autoregulation of inflammation
This story begins almost two decades ago. Barsanjit Mazumder, senior author of this new study, and his colleagues at the time were interested in how our body autoregulates inflammation — not only how it’s initiated but, importantly, how it’s controlled and how it’s turned off once the threat has been cleared. What turns on inflammation is well known. Much about what turns it off remains to be discovered.
Interferon-gamma (IFN-γ) acts both to stimulate and inhibit inflammation, in part by inducing and then silencing the inflammatory protein ceruloplasmin. Ceruloplasmin is produced by macrophages following IFN-γ activation and contributes to the host immune response. As with other inflammatory proteins, ceruloplasmin creates a toxic microenvironment. The flip side of this defensive strategy? It can also end up damaging our own bodies if left unchecked.
Mazumder and his fellow researchers analyzed the potential for interferon-gamma to downregulate ceruloplasmin. They made a startling discovery: IFN-γ-induced extracellular signaling can silence ceruloplasmin mRNA translation, halting its production.
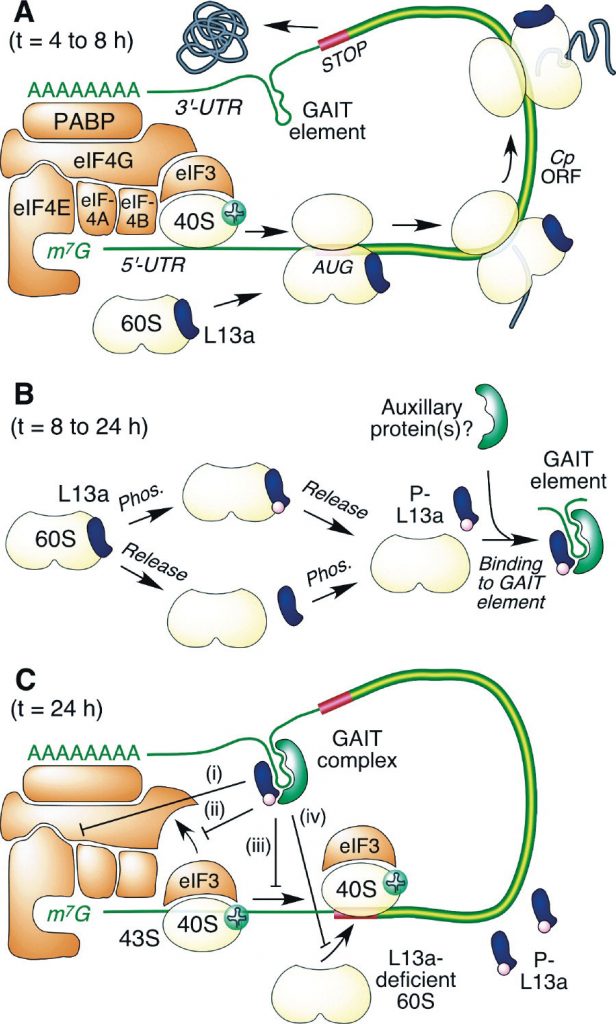
Fourteen to fifteen hours after initial stimulation, IFN-γ activates death-associated protein kinase-1 (DAPK-1). DAPK-1 is a key player in the regulation of apoptosis, a process by which stressed cells commit suicide in such a way as to not induce inflammation; they just quietly digest themselves. DAPK-1, in turn, activates another kinase, zipper-interacting protein kinase (ZIPK), which then phosphorylates a 60S-associated ribosomal protein, L13a.
Once phosphorylated, L13a leaves the ribosome to aggregate with three other proteins — glutamyl-prolyl tRNA synthetase (EPRS), NS1-associated protein 1 (NSAP1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) — to form a complex, the interferon-gamma activated inhibitor of translation (GAIT) complex, or simply GAIT.

A key discovery is that GAIT silences ceruloplasmin synthesis (Figure 1) by binding to a 3 prime stem loop of the mRNA (Figure 2). They named this hairpin RNA structure the GAIT element. We now know that many gamma-interferons are down-regulated by similar GAIT-like elements.
The TGEV GAIT-like element specifically interacts with two host proteins: glutamyl-prolyl-tRNA synthetase (EPRS) and arginyl-tRNA synthetase (RRS). In vitro tests revealed that these proteins directly bind the RNA motif, silencing translation of those mRNAs containing the motif. Almazán et al. engineered a mutant version of the TGEV virus that lacked the GAIT-like RNA motif. The mutant virus elicited a much stronger immune response than did the parent. The authors concluded that the GAIT-like elements act to suppress the host’s immune response.

Spike-ACE2 interactions in SARS-CoV-1
The final hint came from a group of researchers in Taiwan. The cellular protein ACE2 serves as the receptor for both SARS-CoV-1 and -2. Chen et al. noticed that, in some circumstances, binding of ACE2 by the SARS-CoV-1 spike also triggers a kinase cascade altering cellular and viral functions. They found that S protein binding to ACE 2 upregulates the expression of the chemokine ligand 2 (CCL2), a small protein that usually recruits immune cells —monocytes, dendritic cells, and memory T cells— to sites of inflammation. CCL2 is also associated with lung inflammatory disorders including asthma, respiratory distress syndrome, and pulmonary fibrosis. CCL2 knockout mice show a significant reduction in lung fibrosis when compared to their wild-type counterparts.
Chen et al. worked backwards from CCL2 activation to elucidate the signal transduction pathway from S protein binding to gene activation. They determined that activator protein 1 (AP-1) activates the CCL2 promoter upon SARS-CoV-1 infection. AP-1, in turn, is regulated by a set of proteins called mitogen-activated protein kinases (MAPKs). Two of these turned out to be particularly relevant, ERK1 and ERK2. Inhibition of either completely halts CCL2 upregulation.
The full pathway by which S protein ACE2 binding triggers CCL2 production is shown in figure 4. Key players include: casein kinase 2 (CK2) phosphorylation of the cytoplasmic tail of ACE2, the Ras-Raf-MEK-ERK signaling cascade, and AP-1 activation of CCL2 mRNA. Chen et al. suggest that the upregulation of CCL2 by SARS-CoV-1 contributes to SARS lung fibrosis.

S protein inhibition of SARS-CoV-2 messenger RNA expression
Basu et al. noticed that the genome of SARS-CoV-2 has two sequences, one located within ORF1a and a second in the S gene, that could form hairpin loop structures akin to those of canonical GAIT elements (Figures 5 & 6), albeit with dissimilar genetic sequences.

genome. BASU ET AL. 2022
To test the possibility that these sequences also respond to SARS-CoV-2’s spike binding to ACE2, Basu et al. inserted these GAIT-like elements into the 3’ UTR of luciferase mRNA in cell lines that express ACE2. They report that S protein binding to ACE2 silences both luciferase constructs. They also find that S protein presented either on a virus-like particle or on the surface of a lentivirus vector silences constructs carrying either of the two GAIT-like elements. To distinguish these SARS-CoV-2 sequences from their cellular counterparts, they named them “virus-activated inhibitor of translation (VAIT)” elements.

Like GAIT, both SARS-CoV-2 VAIT elements are bound by a multi-protein complex including the phosphorylated L13A protein. Curiously, despite similar secondary stem loop structures, none of the three elements, the ceruloplasmin GAIT element, nor the two SARS-CoV-2 VAIT elements, compete with one another for binding by the L13a complex.
Basu et al. speculate that both the pathway that silences ceruloplasmin and the one that upregulates CCL2 during SARS-CoV-1 infection contribute to the silencing of VAIT element-containing SARS-CoV-2 messenger RNAs. The VAIT pathway is similar to the one that silences ceruloplasmin insofar as they both rely on DAPK-1 kinase to phosphorylate the ribosomal protein L13a. It is similar to the SARS-CoV-1 pathway in its dependence on CK2
and ERK1/2 to kickstart signaling after initial S-ACE2 interaction. Formation of the phosphorylated L13a complex is inhibited either by a drug that blocks DAPK-1 or by DAPK-1 depletion.

Conclusion
These studies show that, independent of its role as an entry receptor for SARS-CoV-1 and -2, extracellular binding of the spike protein to host ACE2 triggers an intracellular cascade that has the potential to alter both cell physiology and expression of viral proteins. The SARS-CoV-1 S protein triggers expression of an active cytokine, CCL2, and S-ACE2 interactions in SARS-CoV-2 may downregulate expression of Orf and S proteins.
An independent study by Alovio et al. found that SARS-CoV-2 spike binding to another receptor, C147, initiates a signaling cascade that disrupts pericyte function and induces death of vascular endothelial cells. Together these studies show that signal transduction induced by sarbecovirus S protein binding to surface receptors are likely to contribute to viral pathogenesis independent of the virus’s ability to enter and to replicate in the target cell.
The post Extracellular Signaling Pathway Inhibits SARS-CoV-2 mRNA Expression appeared first on Inside Precision Medicine.
genome genetic rna coronavirus covid-19International
There will soon be one million seats on this popular Amtrak route
“More people are taking the train than ever before,” says Amtrak’s Executive Vice President.

While the size of the United States makes it hard for it to compete with the inter-city train access available in places like Japan and many European countries, Amtrak trains are a very popular transportation option in certain pockets of the country — so much so that the country’s national railway company is expanding its Northeast Corridor by more than one million seats.
Related: This is what it's like to take a 19-hour train from New York to Chicago
Running from Boston all the way south to Washington, D.C., the route is one of the most popular as it passes through the most densely populated part of the country and serves as a commuter train for those who need to go between East Coast cities such as New York and Philadelphia for business.
Veronika Bondarenko
Amtrak launches new routes, promises travelers ‘additional travel options’
Earlier this month, Amtrak announced that it was adding four additional Northeastern routes to its schedule — two more routes between New York’s Penn Station and Union Station in Washington, D.C. on the weekend, a new early-morning weekday route between New York and Philadelphia’s William H. Gray III 30th Street Station and a weekend route between Philadelphia and Boston’s South Station.
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
According to Amtrak, these additions will increase Northeast Corridor’s service by 20% on the weekdays and 10% on the weekends for a total of one million additional seats when counted by how many will ride the corridor over the year.
“More people are taking the train than ever before and we’re proud to offer our customers additional travel options when they ride with us on the Northeast Regional,” Amtrak Executive Vice President and Chief Commercial Officer Eliot Hamlisch said in a statement on the new routes. “The Northeast Regional gets you where you want to go comfortably, conveniently and sustainably as you breeze past traffic on I-95 for a more enjoyable travel experience.”
Here are some of the other Amtrak changes you can expect to see
Amtrak also said that, in the 2023 financial year, the Northeast Corridor had nearly 9.2 million riders — 8% more than it had pre-pandemic and a 29% increase from 2022. The higher demand, particularly during both off-peak hours and the time when many business travelers use to get to work, is pushing Amtrak to invest into this corridor in particular.
To reach more customers, Amtrak has also made several changes to both its routes and pricing system. In the fall of 2023, it introduced a type of new “Night Owl Fare” — if traveling during very late or very early hours, one can go between cities like New York and Philadelphia or Philadelphia and Washington. D.C. for $5 to $15.
As travel on the same routes during peak hours can reach as much as $300, this was a deliberate move to reach those who have the flexibility of time and might have otherwise preferred more affordable methods of transportation such as the bus. After seeing strong uptake, Amtrak added this type of fare to more Boston routes.
The largest distances, such as the ones between Boston and New York or New York and Washington, are available at the lowest rate for $20.
stocks pandemic japan europeanInternational
The next pandemic? It’s already here for Earth’s wildlife
Bird flu is decimating species already threatened by climate change and habitat loss.

I am a conservation biologist who studies emerging infectious diseases. When people ask me what I think the next pandemic will be I often say that we are in the midst of one – it’s just afflicting a great many species more than ours.
I am referring to the highly pathogenic strain of avian influenza H5N1 (HPAI H5N1), otherwise known as bird flu, which has killed millions of birds and unknown numbers of mammals, particularly during the past three years.
This is the strain that emerged in domestic geese in China in 1997 and quickly jumped to humans in south-east Asia with a mortality rate of around 40-50%. My research group encountered the virus when it killed a mammal, an endangered Owston’s palm civet, in a captive breeding programme in Cuc Phuong National Park Vietnam in 2005.
How these animals caught bird flu was never confirmed. Their diet is mainly earthworms, so they had not been infected by eating diseased poultry like many captive tigers in the region.
This discovery prompted us to collate all confirmed reports of fatal infection with bird flu to assess just how broad a threat to wildlife this virus might pose.
This is how a newly discovered virus in Chinese poultry came to threaten so much of the world’s biodiversity.
The first signs
Until December 2005, most confirmed infections had been found in a few zoos and rescue centres in Thailand and Cambodia. Our analysis in 2006 showed that nearly half (48%) of all the different groups of birds (known to taxonomists as “orders”) contained a species in which a fatal infection of bird flu had been reported. These 13 orders comprised 84% of all bird species.
We reasoned 20 years ago that the strains of H5N1 circulating were probably highly pathogenic to all bird orders. We also showed that the list of confirmed infected species included those that were globally threatened and that important habitats, such as Vietnam’s Mekong delta, lay close to reported poultry outbreaks.
Mammals known to be susceptible to bird flu during the early 2000s included primates, rodents, pigs and rabbits. Large carnivores such as Bengal tigers and clouded leopards were reported to have been killed, as well as domestic cats.
Our 2006 paper showed the ease with which this virus crossed species barriers and suggested it might one day produce a pandemic-scale threat to global biodiversity.
Unfortunately, our warnings were correct.
A roving sickness
Two decades on, bird flu is killing species from the high Arctic to mainland Antarctica.
In the past couple of years, bird flu has spread rapidly across Europe and infiltrated North and South America, killing millions of poultry and a variety of bird and mammal species. A recent paper found that 26 countries have reported at least 48 mammal species that have died from the virus since 2020, when the latest increase in reported infections started.
Not even the ocean is safe. Since 2020, 13 species of aquatic mammal have succumbed, including American sea lions, porpoises and dolphins, often dying in their thousands in South America. A wide range of scavenging and predatory mammals that live on land are now also confirmed to be susceptible, including mountain lions, lynx, brown, black and polar bears.
The UK alone has lost over 75% of its great skuas and seen a 25% decline in northern gannets. Recent declines in sandwich terns (35%) and common terns (42%) were also largely driven by the virus.
Scientists haven’t managed to completely sequence the virus in all affected species. Research and continuous surveillance could tell us how adaptable it ultimately becomes, and whether it can jump to even more species. We know it can already infect humans – one or more genetic mutations may make it more infectious.
At the crossroads
Between January 1 2003 and December 21 2023, 882 cases of human infection with the H5N1 virus were reported from 23 countries, of which 461 (52%) were fatal.
Of these fatal cases, more than half were in Vietnam, China, Cambodia and Laos. Poultry-to-human infections were first recorded in Cambodia in December 2003. Intermittent cases were reported until 2014, followed by a gap until 2023, yielding 41 deaths from 64 cases. The subtype of H5N1 virus responsible has been detected in poultry in Cambodia since 2014. In the early 2000s, the H5N1 virus circulating had a high human mortality rate, so it is worrying that we are now starting to see people dying after contact with poultry again.
It’s not just H5 subtypes of bird flu that concern humans. The H10N1 virus was originally isolated from wild birds in South Korea, but has also been reported in samples from China and Mongolia.
Recent research found that these particular virus subtypes may be able to jump to humans after they were found to be pathogenic in laboratory mice and ferrets. The first person who was confirmed to be infected with H10N5 died in China on January 27 2024, but this patient was also suffering from seasonal flu (H3N2). They had been exposed to live poultry which also tested positive for H10N5.
Species already threatened with extinction are among those which have died due to bird flu in the past three years. The first deaths from the virus in mainland Antarctica have just been confirmed in skuas, highlighting a looming threat to penguin colonies whose eggs and chicks skuas prey on. Humboldt penguins have already been killed by the virus in Chile.

How can we stem this tsunami of H5N1 and other avian influenzas? Completely overhaul poultry production on a global scale. Make farms self-sufficient in rearing eggs and chicks instead of exporting them internationally. The trend towards megafarms containing over a million birds must be stopped in its tracks.
To prevent the worst outcomes for this virus, we must revisit its primary source: the incubator of intensive poultry farms.
Diana Bell does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
genetic pandemic mortality spread deaths south korea south america europe uk chinaUncategorized
NY Fed Finds Medium, Long-Term Inflation Expectations Jump Amid Surge In Stock Market Optimism
NY Fed Finds Medium, Long-Term Inflation Expectations Jump Amid Surge In Stock Market Optimism
One month after the inflation outlook tracked…
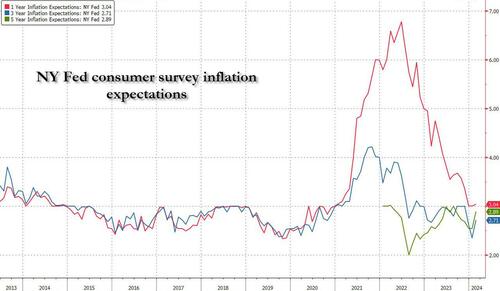
One month after the inflation outlook tracked by the NY Fed Consumer Survey extended their late 2023 slide, with 3Y inflation expectations in January sliding to a record low 2.4% (from 2.6% in December), even as 1 and 5Y inflation forecasts remained flat, moments ago the NY Fed reported that in February there was a sharp rebound in longer-term inflation expectations, rising to 2.7% from 2.4% at the three-year ahead horizon, and jumping to 2.9% from 2.5% at the five-year ahead horizon, while the 1Y inflation outlook was flat for the 3rd month in a row, stuck at 3.0%.
The increases in both the three-year ahead and five-year ahead measures were most pronounced for respondents with at most high school degrees (in other words, the "really smart folks" are expecting deflation soon). The survey’s measure of disagreement across respondents (the difference between the 75th and 25th percentile of inflation expectations) decreased at all horizons, while the median inflation uncertainty—or the uncertainty expressed regarding future inflation outcomes—declined at the one- and three-year ahead horizons and remained unchanged at the five-year ahead horizon.
Going down the survey, we find that the median year-ahead expected price changes increased by 0.1 percentage point to 4.3% for gas; decreased by 1.8 percentage points to 6.8% for the cost of medical care (its lowest reading since September 2020); decreased by 0.1 percentage point to 5.8% for the cost of a college education; and surprisingly decreased by 0.3 percentage point for rent to 6.1% (its lowest reading since December 2020), and remained flat for food at 4.9%.
We find the rent expectations surprising because it is happening just asking rents are rising across the country.
At the same time as consumers erroneously saw sharply lower rents, median home price growth expectations remained unchanged for the fifth consecutive month at 3.0%.
Turning to the labor market, the survey found that the average perceived likelihood of voluntary and involuntary job separations increased, while the perceived likelihood of finding a job (in the event of a job loss) declined. "The mean probability of leaving one’s job voluntarily in the next 12 months also increased, by 1.8 percentage points to 19.5%."
Mean unemployment expectations - or the mean probability that the U.S. unemployment rate will be higher one year from now - decreased by 1.1 percentage points to 36.1%, the lowest reading since February 2022. Additionally, the median one-year-ahead expected earnings growth was unchanged at 2.8%, remaining slightly below its 12-month trailing average of 2.9%.
Turning to household finance, we find the following:
- The median expected growth in household income remained unchanged at 3.1%. The series has been moving within a narrow range of 2.9% to 3.3% since January 2023, and remains above the February 2020 pre-pandemic level of 2.7%.
- Median household spending growth expectations increased by 0.2 percentage point to 5.2%. The increase was driven by respondents with a high school degree or less.
- Median year-ahead expected growth in government debt increased to 9.3% from 8.9%.
- The mean perceived probability that the average interest rate on saving accounts will be higher in 12 months increased by 0.6 percentage point to 26.1%, remaining below its 12-month trailing average of 30%.
- Perceptions about households’ current financial situations deteriorated somewhat with fewer respondents reporting being better off than a year ago. Year-ahead expectations also deteriorated marginally with a smaller share of respondents expecting to be better off and a slightly larger share of respondents expecting to be worse off a year from now.
- The mean perceived probability that U.S. stock prices will be higher 12 months from now increased by 1.4 percentage point to 38.9%.
- At the same time, perceptions and expectations about credit access turned less optimistic: "Perceptions of credit access compared to a year ago deteriorated with a larger share of respondents reporting tighter conditions and a smaller share reporting looser conditions compared to a year ago."
Also, a smaller percentage of consumers, 11.45% vs 12.14% in prior month, expect to not be able to make minimum debt payment over the next three months
Last, and perhaps most humorous, is the now traditional cognitive dissonance one observes with these polls, because at a time when long-term inflation expectations jumped, which clearly suggests that financial conditions will need to be tightened, the number of respondents expecting higher stock prices one year from today jumped to the highest since November 2021... which incidentally is just when the market topped out during the last cycle before suffering a painful bear market.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex



























