Uncategorized
David Ricks, Eli Lilly CEO, on development of coronavirus therapies
David Ricks, Eli Lilly CEO, on development of coronavirus therapies


CNBC Transcript: Eli Lilly CEO David Ricks speaks with CNBC’s “Squawk on the Street” today on development of coronavirus therapies
WHEN: Today, Monday, March 30, 2020
WHERE: CNBC’s “Squawk on the Street”
The following is the unofficial transcript of a CNBC interview with Eli Lilly CEO David Ricks, Former Metronic CEO and CNBC Contributor Bill George, and CNBC’s “Squawk on the Street” (M-F 9AM – 11AM) today, Monday, March 30th. The following is a link to video of the interview on CNBC.com:
Q4 2019 hedge fund letters, conferences and more
Eli Lilly CEO David Ricks On Development Of Coronavirus Therapies
All references must be sourced to CNBC.
CARL QUINTANILLA: Meantime, guys, Eli Lilly is co-developing a potential treatment for COVID-19 with private biotech firm AbCellera. We're joined this morning by Eli Lilly’s Chairman and CEO David Ricks, as well as former Medtronic CEO and CNBC contributor Bill George. Guys, good to see you both. Bill, let me turn this over to you and start us off.
BILL GEORGE: Good morning. Dave, thank you very much for being here. We can only imagine just how busy you are trying to keep the company running at this difficult time, with so many people working from home. So, you announced this partnership with AbCellera to go in the direction of finding a treatment with antibodies. And yet, many of your competitors like Alex Gorksy was just on talking about a vaccine starting up in September and Moderna is already in the clinic. Tell us why you went the route of Coronavirus antibody therapies to find a therapy for COVID-19, and how you're coming on it. I realize it's only been two weeks since you made the announcement but what's your outlook?
DAVID RICKS: Right. Yeah, well, thanks for having me on. And maybe just to start, as well, you know, today is National Doctors Day, and from our company to every doctor in the U.S. and I'm sure from all of you in the studio, we want to thank our frontline health care workers right now. This is a serious moment for all of them, and we're all thinking of them as they go to their, their job today to save lives, which is what they trained to do. We're trying to do our part too. This is, you mentioned the AbCellera therapeutics collaboration we have. And I think across the whole industry, Bill, many companies, maybe all companies are redirecting all discretionary scientific and medical prowess at the problem of arresting the coronavirus. For us, that falls into two tracks one of them you mentioned, which is the AbCellera therapeutics collaboration.
We're good at making antibodies and scaling them up and producing them and modifying them to be more like drugs. What AbCellera has done is isolated antibodies from actual human survivors of COVID-19 and have selected antibodies that appear to have a strong effect on arresting the spread of the virus. We're going to try to isolate those and make them into a one, two or three antibody-drug cocktail. We hope to start clinical trials as early as July. Other companies are doing different things, making vaccines, or repurposing existing treatments.
We've heard about antivirals and some anti-inflammatory drugs. We have one of those projects too, with an anti-inflammatory drug which is being tested by clinical investigators around the world called Baricitinib to help those who have ARDS, the acute respiratory distress syndrome. That's another approach. So, but across the industry, everybody is working together, and in a very rapid way to try to arrest this crisis.
BILL GEORGE: So, Dave just to follow up on that. Yesterday, the FDA announced the approval for Anti-Malarial drugs without any clinical trial. Work done, even though Vas Narasimhan was on last week saying could be a year and a half to two. If they're promising results for the Coronavirus antibody therapies, is there a possibility that we might truncate the usual, year and a half or so of clinical trials and get there sooner? Is there a way to get there faster or is it pretty much going to take that long?
DAVID RICKS: Well, we hope so. And I think every signal we’ve received from the FDA and other government agencies is, is they're moving at lightspeed as well. And we'd like to see this tested in patients first we need to do clinical experiment, but that won't take too long, and then potentially look at either a very large clinical trial to expose people who are sick and tested in the real-life, or even a conditional license type arrangement where we can make it available. Interestingly, you know maybe that constraint might be, how much we can produce. And we're pulling forward activities and planning around that so that we can make enough if it does work. So, we're planning for the fastest possible route and the FDA is moving on these anti-malarial signals, I think their posture, which is to open up all aperture for solutions.
JIM CRAMER: Hey David. It's always great to see you. It's Jim.
DAVID RICKS: Hey, Jim.
JIM CRAMER: You mentioned something that is the holy grail. You're working on something for ARDS. ARDS is just notoriously difficult. It's what's killing a lot of people. It killed my father. And when I was investigating this, as obviously you would as a son. I was told this was it was at -- forget about it, Jim, there's nothing for ARDS. Do you really think you have something?
DAVID RICKS: Well, so there's a number of experts in immunology who believe you know the ARDS happens in COVID-19 because of the response to the virus itself your body's own immune system is flaring up and producing a huge amount of response in your lungs to the virus. This is actually one of the main problems people have with oxygen exchange and breathing in the advanced stage of the disease. There are studies already announced with the so-called aisle six inhibitors called Actemra and Kevzara from two other drug companies. This is thought to be a promising approach. If that works, the approach we're looking at likely also will make a difference. Because it works in a similar way reducing that inflammatory response in your own lungs, which could keep people from going on to ventilators or get them off ventilation quicker. And as I said, Jim, there are studies in China, in Europe and in the U.S., that are investigator-initiated now with our drug Baricitinib looking at this problem. We'll talk more about that as we see the data, and we may decide to stand up an independent study ourselves.
JIM CRAMER: What a wonder drug. Thank you, David. What a wonderful drug.
CARL QUINTANILLA: David it's Carl. I hate to ask about father on Mother's Day, but you do mention some of the different paths that pharma companies are taking. And it does feel like our viewers have received an inordinate amount of opinion about hydroxychloroquine. What do you tell people who are curious about it?
DAVID RICKS: Well it does have some preclinical effects. That's what our scientists have advised me. And there's a number of small studies that have been uncontrolled that look promising. You know, in our business typically we like to do a randomized control study to really know whether something is making a difference. We haven't seen that data yet here. That said, I think the federal agencies have a different job than us.
Our job is to prove effectiveness. Their job is to protect and promote the public health. That is the FDA’s mission. And their judgment at this point is, is to move forward with less evidence apparently. That's their call to make. I do think that we would all benefit from a randomized control study being done with this medicine to see the true effect. I don't know that would take as long as the number Bill mentioned earlier, a year or two.
For our approach with accelerating this therapeutic antibody approach we're planning for a relatively short study because if it works, it'll work very quickly. One would hope if it's really an antiviral effect with the malaria drugs that that would be quick as well.
SARA EISEN: You know David, I, the history here is not lost on a lot of people. You were the first in the U.S. to mass-produce the polio vaccine. You gave us insulin. Do you see this as a moment for you and for your company to be the first to really give us the good news that we're hoping for, a medication against COVID-19? Where do you stand against the so many other treatments that are out there in testing?
DAVID RICKS: Well, I think, first of all, I think historically the company, we're an old company, we've been a part of those kinds of medical breakthroughs. For weeks ago, I had a conversation with my Chief Scientific Officer, the answer -- and we said put every discretionary effort on COVID-19. We're not a viral company. We have no core expertise there. But we have expertise in creating antibodies, in autoimmunity. We talked about Baricitinib. We've redirected a huge part of our laboratory work actually to testing people in Indianapolis. Because there was a huge testing shortage here. So, we created our own test. We had it certified and are testing about 1000 people a day at our corporate center hear from the community. So, this is an all hands on deck response. To me it doesn't matter if Lilly is the one who has the breakthrough or Regeneron or you mentioned Johnson & Johnson or anyone else. What matters, there is one, and it happens quickly, but what I can tell, viewers is, working across the industry talking to other CEOs in the sector, I have never seen a moment like this where everybody is sharing information collaborating and working with the kind of urgency we have now. Hopefully, that will result in a quick response from one of the companies, and hopefully, we can get back to life as we know it, pre, pre the coronavirus. Certainly, everyone's intention is to get to that point as quickly as we can. And if it's if it's a Lilly effort that has a breakthrough, so be it. But right now, those individual credit components don't matter. Nobody's talking about credit, nobody's talking about profit, we're all just trying to get an answer to this humanitarian crisis.
BILL GEORGE: Dave, right now we're still seeing -- meanwhile, before all, we come up with a solution, we're seeing a fast ramp-up it's even growing on a logarithmic basis right now. And we need help for people and obviously the most at-risk group for people with comorbidities and probably the leading comorbidity right now is diabetes and you're the leading insulin producer and a lot of people are concerned about getting insulin. They're concerned about whether they can afford it. I happen to notice you had this in the morning paper, you had a full-page ad. What are you going to do for the insulin sufferers, particularly people who have lost their job, and don't have the money to pay for their insulin right now but desperately need it to avoid getting sick?
DAVID RICKS: Yeah, thanks for raising that Bill today we did launch a national print campaign and we're trying to raise awareness about what we call our Diabetes Solutions Center. This is a phone number, or you can go to Lilly.com if you have diabetes and use insulin, where we provide health financial assistance to people who are having trouble bridging the gap. Whether you have insurance, and you don't have enough money to afford your part of the insulin prescription, or you've lost your insurance, this is, we have solutions for you. So please, I encourage viewers to call in and that's what this campaign is aimed at. We have two important jobs as a leading insulin producer. One is to make the insulin. And I'm so proud of our workers who come to our plant every day here in Indiana. We make it in the U.S. And they're coming day and night to make insulin so there is no gap and supply we have plenty of supply. And our job first is to make sure we supply lifesaving medicines. The other job, though, is to make sure people can actually get them at the pharmacy counter. And we know the patchwork of American healthcare can be difficult to navigate that people do struggle with affordability, but what I'm here to say today is we have answers for almost everybody in the U.S. healthcare system if you're having trouble paying for insulin. And I encourage people to go to Lilly.com and connect to our resource center. It only takes about 10 minutes on average per patient, we have bilingual operators there, and we can help you. And that's what we announced today with, with the print advertisement. And actually, that's been running for a year and a half, but we wanted to reinforce the value of that resource for patients in this difficult time.
DAVID FABER: David it's David Faber when you joined Jim on Mad Money on March 13 you said, it may take more than one medicine to recover from coronavirus. Is that still your belief?
DAVID RICKS: Yes, I think once you have – you’re becoming hospitalized, you have a respiratory response that is no longer manageable by the individual, it may take several medicines. In fact, most real disease advancements we've seen in recent years have been combinations of approaches. And here we've talked about anti-inflammatory approaches. We've talked about using antibodies to attack the virus directly, and there could be retrovirals or other small molecules pills that could address this. And a cocktail of approaches may be used. In fact, patients are managed today with a cocktail of approaches in the ICU, just none of them are directly attacking the virus. So, I do think so and that's, again, one of the benefits of this all hands on deck pan-industry effort to come up with solutions we may come up with several that together could have a profound impact.
BILL GEORGE: David you've had a remarkable transformation this short period of time. I understand that you shut down all your other clinical trials for this period. You’ve suspended, not shut down but suspended them, and you're doing this drive up testing 1000 tests a day for people in Indiana. But how are you keeping the company running with everyone working at home, and trying to get people to come to your factories to work, many must have children at home with the school shut down and give a sense inside of how you keep the place running in this time as a leader and how you keep it going, how you give people the courage to keep going up the hill?
DAVID RICKS: Yeah, Bill, thanks. It certainly has been an experiment we've never – we had Business Continuity Planning but never thought about the scale of this, running a global company off the internet, as it were. But that is in fact what we're doing. Of course, our plants where we make products and that's as I said our most fundamental responsibility is keep product flowing, particularly insulin, as you mentioned, they're operating and workers are coming in as we request them. We've given shift premiums and extra resources to those employees. Because I think they're giving up a lot coming to make products for patients.
But they know that's our purpose and our responsibility for basically every other type of work we are working remotely. We've adopted technology that we had been dabbling in but now we're running at massive scale. We use a lot of Microsoft suite of tools for corporate America and they work pretty well. I have to say, we never thought we'd have 30,000 people dialing in at the same time to various activities, but they are and it works.
So, I heard the Livongo CEO earlier and I think he's right that in many parts of our society, there's going to be a reset toward more remote way of working, and habits will likely not go back. One of them could be more flexibility for corporate America workers to work from home when they need to or come into the office when they need to, hopefully when we get back to a more regular pattern, people will then have more choices and that would be a good thing. In terms of childcare, we have childcare on-site and those are still open for employees, particularly those manufacturing workers who come in so they can get assistance that way as well.
CARL QUINTANILLA: David, our thanks to you for all the information and guidance that you've been giving us. And please come back as often as you can. David Rick of Lilly. and Bill George, our thanks to you.
The post David Ricks, Eli Lilly CEO, on development of coronavirus therapies appeared first on ValueWalk.
Uncategorized
Homes listed for sale in early June sell for $7,700 more
New Zillow research suggests the spring home shopping season may see a second wave this summer if mortgage rates fall
The post Homes listed for sale in…
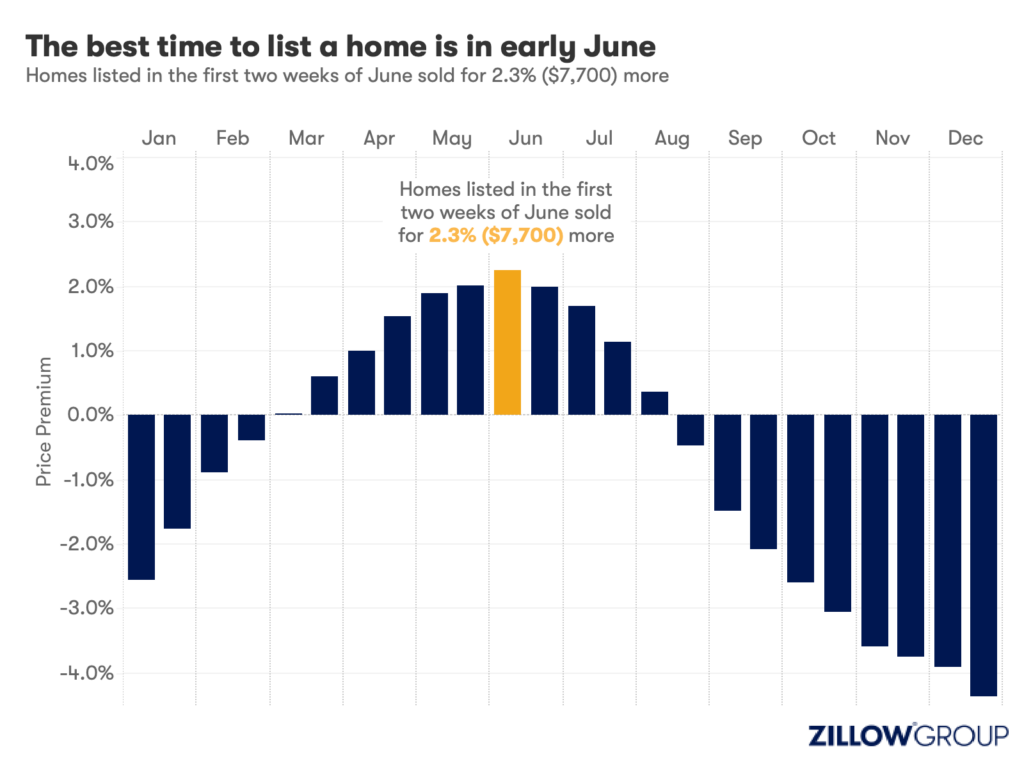
- A Zillow analysis of 2023 home sales finds homes listed in the first two weeks of June sold for 2.3% more.
- The best time to list a home for sale is a month later than it was in 2019, likely driven by mortgage rates.
- The best time to list can be as early as the second half of February in San Francisco, and as late as the first half of July in New York and Philadelphia.
Spring home sellers looking to maximize their sale price may want to wait it out and list their home for sale in the first half of June. A new Zillow® analysis of 2023 sales found that homes listed in the first two weeks of June sold for 2.3% more, a $7,700 boost on a typical U.S. home.
The best time to list consistently had been early May in the years leading up to the pandemic. The shift to June suggests mortgage rates are strongly influencing demand on top of the usual seasonality that brings buyers to the market in the spring. This home-shopping season is poised to follow a similar pattern as that in 2023, with the potential for a second wave if the Federal Reserve lowers interest rates midyear or later.
The 2.3% sale price premium registered last June followed the first spring in more than 15 years with mortgage rates over 6% on a 30-year fixed-rate loan. The high rates put home buyers on the back foot, and as rates continued upward through May, they were still reassessing and less likely to bid boldly. In June, however, rates pulled back a little from 6.79% to 6.67%, which likely presented an opportunity for determined buyers heading into summer. More buyers understood their market position and could afford to transact, boosting competition and sale prices.
The old logic was that sellers could earn a premium by listing in late spring, when search activity hit its peak. Now, with persistently low inventory, mortgage rate fluctuations make their own seasonality. First-time home buyers who are on the edge of qualifying for a home loan may dip in and out of the market, depending on what’s happening with rates. It is almost certain the Federal Reserve will push back any interest-rate cuts to mid-2024 at the earliest. If mortgage rates follow, that could bring another surge of buyers later this year.
Mortgage rates have been impacting affordability and sale prices since they began rising rapidly two years ago. In 2022, sellers nationwide saw the highest sale premium when they listed their home in late March, right before rates barreled past 5% and continued climbing.
Zillow’s research finds the best time to list can vary widely by metropolitan area. In 2023, it was as early as the second half of February in San Francisco, and as late as the first half of July in New York. Thirty of the top 35 largest metro areas saw for-sale listings command the highest sale prices between May and early July last year.
Zillow also found a wide range in the sale price premiums associated with homes listed during those peak periods. At the hottest time of the year in San Jose, homes sold for 5.5% more, a $88,000 boost on a typical home. Meanwhile, homes in San Antonio sold for 1.9% more during that same time period.
| Metropolitan Area | Best Time to List | Price Premium | Dollar Boost |
| United States | First half of June | 2.3% | $7,700 |
| New York, NY | First half of July | 2.4% | $15,500 |
| Los Angeles, CA | First half of May | 4.1% | $39,300 |
| Chicago, IL | First half of June | 2.8% | $8,800 |
| Dallas, TX | First half of June | 2.5% | $9,200 |
| Houston, TX | Second half of April | 2.0% | $6,200 |
| Washington, DC | Second half of June | 2.2% | $12,700 |
| Philadelphia, PA | First half of July | 2.4% | $8,200 |
| Miami, FL | First half of June | 2.3% | $12,900 |
| Atlanta, GA | Second half of June | 2.3% | $8,700 |
| Boston, MA | Second half of May | 3.5% | $23,600 |
| Phoenix, AZ | First half of June | 3.2% | $14,700 |
| San Francisco, CA | Second half of February | 4.2% | $50,300 |
| Riverside, CA | First half of May | 2.7% | $15,600 |
| Detroit, MI | First half of July | 3.3% | $7,900 |
| Seattle, WA | First half of June | 4.3% | $31,500 |
| Minneapolis, MN | Second half of May | 3.7% | $13,400 |
| San Diego, CA | Second half of April | 3.1% | $29,600 |
| Tampa, FL | Second half of June | 2.1% | $8,000 |
| Denver, CO | Second half of May | 2.9% | $16,900 |
| Baltimore, MD | First half of July | 2.2% | $8,200 |
| St. Louis, MO | First half of June | 2.9% | $7,000 |
| Orlando, FL | First half of June | 2.2% | $8,700 |
| Charlotte, NC | Second half of May | 3.0% | $11,000 |
| San Antonio, TX | First half of June | 1.9% | $5,400 |
| Portland, OR | Second half of April | 2.6% | $14,300 |
| Sacramento, CA | First half of June | 3.2% | $17,900 |
| Pittsburgh, PA | Second half of June | 2.3% | $4,700 |
| Cincinnati, OH | Second half of April | 2.7% | $7,500 |
| Austin, TX | Second half of May | 2.8% | $12,600 |
| Las Vegas, NV | First half of June | 3.4% | $14,600 |
| Kansas City, MO | Second half of May | 2.5% | $7,300 |
| Columbus, OH | Second half of June | 3.3% | $10,400 |
| Indianapolis, IN | First half of July | 3.0% | $8,100 |
| Cleveland, OH | First half of July | 3.4% | $7,400 |
| San Jose, CA | First half of June | 5.5% | $88,400 |
The post Homes listed for sale in early June sell for $7,700 more appeared first on Zillow Research.
federal reserve pandemic home sales mortgage rates interest ratesUncategorized
February Employment Situation
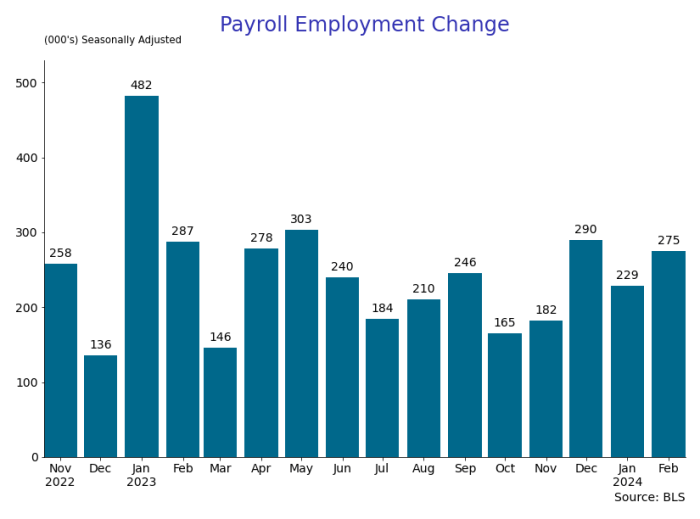
By Paul Gomme and Peter Rupert The establishment data from the BLS showed a 275,000 increase in payroll employment for February, outpacing the 230,000…
By Paul Gomme and Peter Rupert
The establishment data from the BLS showed a 275,000 increase in payroll employment for February, outpacing the 230,000 average over the previous 12 months. The payroll data for January and December were revised down by a total of 167,000. The private sector added 223,000 new jobs, the largest gain since May of last year.

Temporary help services employment continues a steep decline after a sharp post-pandemic rise.


Average hours of work increased from 34.2 to 34.3. The increase, along with the 223,000 private employment increase led to a hefty increase in total hours of 5.6% at an annualized rate, also the largest increase since May of last year.

The establishment report, once again, beat “expectations;” the WSJ survey of economists was 198,000. Other than the downward revisions, mentioned above, another bit of negative news was a smallish increase in wage growth, from $34.52 to $34.57.

The household survey shows that the labor force increased 150,000, a drop in employment of 184,000 and an increase in the number of unemployed persons of 334,000. The labor force participation rate held steady at 62.5, the employment to population ratio decreased from 60.2 to 60.1 and the unemployment rate increased from 3.66 to 3.86. Remember that the unemployment rate is the number of unemployed relative to the labor force (the number employed plus the number unemployed). Consequently, the unemployment rate can go up if the number of unemployed rises holding fixed the labor force, or if the labor force shrinks holding the number unemployed unchanged. An increase in the unemployment rate is not necessarily a bad thing: it may reflect a strong labor market drawing “marginally attached” individuals from outside the labor force. Indeed, there was a 96,000 decline in those workers.


Earlier in the week, the BLS announced JOLTS (Job Openings and Labor Turnover Survey) data for January. There isn’t much to report here as the job openings changed little at 8.9 million, the number of hires and total separations were little changed at 5.7 million and 5.3 million, respectively.

As has been the case for the last couple of years, the number of job openings remains higher than the number of unemployed persons.

Also earlier in the week the BLS announced that productivity increased 3.2% in the 4th quarter with output rising 3.5% and hours of work rising 0.3%.

The bottom line is that the labor market continues its surprisingly (to some) strong performance, once again proving stronger than many had expected. This strength makes it difficult to justify any interest rate cuts soon, particularly given the recent inflation spike.
unemployment pandemic unemploymentUncategorized
Mortgage rates fall as labor market normalizes
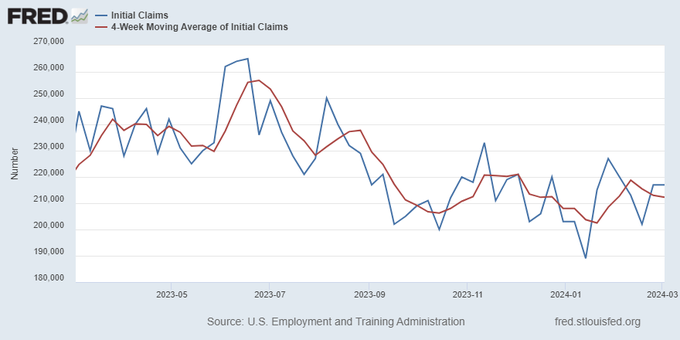
Jobless claims show an expanding economy. We will only be in a recession once jobless claims exceed 323,000 on a four-week moving average.
Everyone was waiting to see if this week’s jobs report would send mortgage rates higher, which is what happened last month. Instead, the 10-year yield had a muted response after the headline number beat estimates, but we have negative job revisions from previous months. The Federal Reserve’s fear of wage growth spiraling out of control hasn’t materialized for over two years now and the unemployment rate ticked up to 3.9%. For now, we can say the labor market isn’t tight anymore, but it’s also not breaking.
The key labor data line in this expansion is the weekly jobless claims report. Jobless claims show an expanding economy that has not lost jobs yet. We will only be in a recession once jobless claims exceed 323,000 on a four-week moving average.
From the Fed: In the week ended March 2, initial claims for unemployment insurance benefits were flat, at 217,000. The four-week moving average declined slightly by 750, to 212,250
Below is an explanation of how we got here with the labor market, which all started during COVID-19.
1. I wrote the COVID-19 recovery model on April 7, 2020, and retired it on Dec. 9, 2020. By that time, the upfront recovery phase was done, and I needed to model out when we would get the jobs lost back.
2. Early in the labor market recovery, when we saw weaker job reports, I doubled and tripled down on my assertion that job openings would get to 10 million in this recovery. Job openings rose as high as to 12 million and are currently over 9 million. Even with the massive miss on a job report in May 2021, I didn’t waver.
Currently, the jobs openings, quit percentage and hires data are below pre-COVID-19 levels, which means the labor market isn’t as tight as it once was, and this is why the employment cost index has been slowing data to move along the quits percentage.

3. I wrote that we should get back all the jobs lost to COVID-19 by September of 2022. At the time this would be a speedy labor market recovery, and it happened on schedule, too
Total employment data
4. This is the key one for right now: If COVID-19 hadn’t happened, we would have between 157 million and 159 million jobs today, which would have been in line with the job growth rate in February 2020. Today, we are at 157,808,000. This is important because job growth should be cooling down now. We are more in line with where the labor market should be when averaging 140K-165K monthly. So for now, the fact that we aren’t trending between 140K-165K means we still have a bit more recovery kick left before we get down to those levels.

From BLS: Total nonfarm payroll employment rose by 275,000 in February, and the unemployment rate increased to 3.9 percent, the U.S. Bureau of Labor Statistics reported today. Job gains occurred in health care, in government, in food services and drinking places, in social assistance, and in transportation and warehousing.
Here are the jobs that were created and lost in the previous month:

In this jobs report, the unemployment rate for education levels looks like this:
- Less than a high school diploma: 6.1%
- High school graduate and no college: 4.2%
- Some college or associate degree: 3.1%
- Bachelor’s degree or higher: 2.2%

Today’s report has continued the trend of the labor data beating my expectations, only because I am looking for the jobs data to slow down to a level of 140K-165K, which hasn’t happened yet. I wouldn’t categorize the labor market as being tight anymore because of the quits ratio and the hires data in the job openings report. This also shows itself in the employment cost index as well. These are key data lines for the Fed and the reason we are going to see three rate cuts this year.
recession unemployment covid-19 fed federal reserve mortgage rates recession recovery unemployment-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex




