Biotech Bonanza: The M&A Theme
Biotech Bonanza: The M&A Theme
- Biotechs have led the V-shaped market decline and recovery over the past 2 months
- A rise in M&A activity after a moribund 2018 has helped the sector to recover faster and positions it for further gains
- Low in-house R&D productivity and pressure on drug pricing create a difficult environment for pharmaceuticals and another reason for M&A
- Drug pricing regulation and greater political focus can hold back sector returns, although there will be many opportunities to outperform indexes.
- A portfolio comprising biotech companies with a promising pipeline will benefit from enhanced industry valuation if the M&A push is sustained.
Biotech Pulse

Biotech tumbled off a steep ski slope in the last quarter and at the December low the Nasdaq Biotechnology Index (IBB) had declined -28%, while the S&P Biotech Select Index (XBI) a whopping -36%. But it seems biotechs were able to regain their composure at the lows and ski gracefully higher during January in what now appears to have been a V-shaped two-month ride. Most likely, the pace of sharp run-up will now moderate out as it has offset the panic selling during December. In addition, the growing presence of familiar headwinds can prove to be a drag on the biopharma industries. Nonetheless, biotechs will continue to provide opportunities for robust gains.
Timely M&A Restores Confidence
In the Biotech Outlook 2019, published last December, we had mentioned that:
"Predicting a biopharma M&A ramp-up has become just as hard as predicting the results of a drug trial - totally uncertain...but, lower valuations may spark activity."
It was quite encouraging to see M&A activity in the biotechnology sector tick up measurably, right at the time when the sector needed a confidence booster. The rise in such activity to a significant extent explains why the biotech sector has led the market in the January recovery.
The biggest acquisition highlight was Bristol-Myers Squibb's (BMY) move to acquire biotechnology behemoth, Celgene (CELG). This was an acquisition of one of the top 4 biotech companies by market valuations. Only Gilead (GILD), Amgen (AMGN), and Biogen (BIIB) are bigger. There has not been this big a biotech acquisition for a very long time. Perhaps the market selloff in December contributed towards the final push to pull the trigger by Bristol on the $74 billion acquisition. The transaction did magic for the biotech sector and provided additional fuel to an emerging recovery.
A couple of days later, Eli Lilly (ELY) announced the acquisition of Loxo Oncology (LOXO) for $8 billion in cash, further sparking investor enthusiasm for biotechs.
Biotech M&A - The Anyday Now Narrative May Come True
Acquisitions have remained a viable theme for the biotech sector for some time now driven by a number of compelling reasons, which include the lower R&D return for big pharmaceutical companies, the sizable cash holdings, and a promising and well-developed pool of target biotech companies further boosted by a record 56 IPOs in 2018. However, the transaction results have been disappointing in 2017 and 2018, as seen in the M&A activity table below.
The only reason 2018 showed an improvement over 2017 was due to a single large $64 billion acquisition of Shire Pharmaceuticals by Takeda.
With over $80 billion of deals announced in January 2019, it is not hard to envision that 2019 appears to be well-positioned to significantly surpass 2018 transaction value.
There are a number of companies whose names have been put forward as potential targets over the past year, and some of them quite frequently for a long time. A few of these include: Alexion Pharmaceuticals (ALXN) and Incyte (INCY) as large-cap biotech targets, particularly after the acquisition of Celgene; Clovis Oncology (CLVS) for its PARP-inhibitor, since Tesaro (TSRO) has now finally found a new home at GlaxoSmithKline (GSK); Amarin (AMRN) for its Vascepa rollout; Intercept Pharmaceuticals (ICPT) for its late-stage NASH asset, while Madrigal Pharmaceuticals (MDGL) and Viking Therapeutics (VKTX) for their mid-and-early-stage NASH products; Esperion Therapeutics (ESPR) and The Medicines Company (MDCO) for their cholesterol management late-stage assets; Acadia Pharmaceuticals (ACAD), Sarepta Therapeutics (SRPT), Heron Therapeutics (HRTX), which have been talked about as candidates for years like many others.
There are obviously many more companies which can fit the profile of an attractive target, and readers may build on this abbreviated list further in the Comments section.
There are a number of companies whose names have been put forward as potential targets over the past year, and some of them quite frequently for a long time. A few of these include: Alexion Pharmaceuticals (ALXN) and Incyte (INCY) as large-cap biotech targets, particularly after the acquisition of Celgene; Clovis Oncology (CLVS) for its PARP-inhibitor, since Tesaro (TSRO) has now finally found a new home at GlaxoSmithKline (GSK); Amarin (AMRN) for its Vascepa rollout; Intercept Pharmaceuticals (ICPT) for its late-stage NASH asset, while Madrigal Pharmaceuticals (MDGL) and Viking Therapeutics (VKTX) for their mid-and-early-stage NASH products; Esperion Therapeutics (ESPR) and The Medicines Company (MDCO) for their cholesterol management late-stage assets; Acadia Pharmaceuticals (ACAD), Sarepta Therapeutics (SRPT), Heron Therapeutics (HRTX), which have been talked about as candidates for years like many others.
A recent analysis by Evaluate Pharma revealed that based on the historical data over the past 5 years, a majority of the buyouts have occurred for products in the Pre-clinical and Phase II stages.
The lower number of Phase III buyouts is understandable as such assets are also more scarce, besides being at a higher valuation, compared to the volume of assets available in Pre-clinical and Phase II.
The analysis also indicated that the leading therapy area for buyouts remains Oncology, as it has been for many years.
The recent slump in activity has not only been limited to M&A transactions but even licensing deals, which hit a five-year low in 2018.
The strong start to M&A activity in 2019, if it persists, may also contribute to spurring greater licensing activity this year.
However, buying a company just for its acquisition appeal can be a frustrating experience and even an ineffective portfolio strategy. A more prudent strategy would be to purchase promising companies for their product pipeline and expect M&A activity to lift valuations across the sector. In such a case, one may even get fortunate enough then to hold a company that gets acquired.
New Product Launches And Key Data Releases
The advancement of scientific frontiers has opened up new areas of exploration and opportunity to create potential blockbuster cures. This has attracted significant amounts of capital as evidenced by the number of biotechs receiving venture funding and the biotech IPOs - both setting records last year.
2019 is shaping up strongly for new data releases which can contribute towards a positive sector glow. In fact, the period 2019-2023 will have more new active substances or new drugs launched per year than at any time over a 5-year period since 2004.
As the above exhibit shows, on average 54 new drugs are expected to be launched per year for the next 5 years, compared to 46 drugs for the prior 5-year period.
In 2019 there are some key product launches, notably Alexion Pharmaceuticals' Ultomiris drug for rare blood disorder which was approved last December, and Kala Pharmaceuticals' Inveltys for post-surgery eye pain and inflammation.
Below is a table of a few key data releases and product launches.
2019 - Few Key Launches and Approval Decisions
| Product | Company | Event | 2024 Sales Est ($bn) | |
| 1 | Ultomiris | Alexion Pharmaceuticals | Approved Dec 2018 | 3.4 |
| 2 | Risankizumab | Abbvie | PDUFA Apr 25, 2019 | 2.2 |
| 3 | LentiGlobin | Bluebird Bio | EU - due Q2, 2019 | 1.9 |
| 4 | Zolgensma | Novartis | FDA - due May 2019 | 1.6 |
| 5 | Inveltys | Kala Pharmaceutical (KALA) | Launched Jan 2019 | 1.4 |
| 6 | Esketamine | Johnson & Johnson (JNJ) | PDUFA May 3, 2019 | 1.3 |
| 7 | Siponimod | Novartis (NVS) | FDA - due Mar 2019 | 1.3 |
| 8 | Selinexor | Karyopharm (KPTI) | PDUFA April 5, 2019 | 1.2 |
| 9 | Zanubrutinib | Beigene (BGNE) | China approval expected mid-2019 | 1.2 |
| 10 | Lumateperone | Intra-Cellular Therapies | PDUFA Sep 27,2019 | 1.0 |
| 11 | Roxadustat | Astrazeneca (AZN) / Astellas / Fibrogen (FGEN) | US/EU filing expected H1'19 | 1.9 |
Source: EvaluatePharma
Much of the drug innovation is happening in the smallcap and midcap space with companies that have revenues of less than $500 million as shown in the graphic below.
This further strengthens the argument supporting M&A in the small-and-midcap space as the pharmaceutical companies strive to expand their pipelines with new compounds.
Regulatory Landscape To Become Noisier
As discussed in our 2019 outlook article, it is quite clear that with a Democrat control of the House, a President itching for a headline achievement on drug price reduction, and a warming-up battle for Presidential nomination, healthcare will receive more share of political attention than desired by the industry. The healthcare (XLV) sector faces the risk of constantly evolving regulations. However, the confluence of the above events creates a higher risk profile for pharmaceutical and biotech companies, similar to the one evidenced during 2015 and 2016, as the threat of drug price regulation remains a persistent backdrop to industry fundamentals. The biotech industry depends on the pharmaceutical industry for its eventual high-valuation exit. So what's good for the pharma industry is often good for biotechs.
The House Committee on Oversight and Reform showed the priority of the drug pricing issue in its agenda by holding its first hearing of the 116th Congress on the subject of prescription drug prices. The President's remarks at the State of the Union on lowering drug prices were quite clear as well. Half-a-dozen bills have been introduced just over the past few months to target drug pricing using different approaches.
Drug cost is an issue that resonates strongly with Americans and consistently ranks as the biggest health care concern. It is an issue with decent bipartisan support, and that itself is notable in this divided Congress.
Could there be a grand bargain between the President and the Democrats on this issue?
It's possible. But at this juncture unlikely as the Democrats launch multiple investigations on the President's activities which may create an even more uncompromising environment in the government. Any other proposed healthcare legislation by the House will need Republican support in the Senate. That's possible but again a low probability outcome.
So most of the bills may not end up as legislation and be just noise. But, the hearings and the process can still be quite uncomfortable for the industry and investors.
In this debate, it will be helpful to also understand the cost of developing a drug for large pharmaceutical and biotech companies. It is well-known that for each drug that is approved, the number of drugs that end up failing is many times over. So the average cost of a single approved drug to a large pharmaceutical company can be viewed as the size of their R&D budget (success + failures) over the years spread over the number of approved drugs. Data indicates that an approved new drug can potentially cost $6 billion after looking at the spend on R&D failures, as we discuss further in the next section.

Pharmaceutical Companies In A Pincer
The pharmaceutical companies are in a pincer, caught between lower R&D productivity and rising backlash on prices.
On the one hand, they need to make aggressive moves to create new product lines to prepare for the day when their blockbuster franchises begin to erode. For some pharma, such a day is approaching fast if not already here. While on the other hand, the massive in-house R&D expenditures are not delivering a sufficient return on the capital being deployed.
Recently, an interesting statistic caught our eye in an article by Idea Pharma, a pharmaceutical consulting company, about the estimated cost of a drug. The firm maintains a Pharma Innovation Index and used the data for the top 11, out of 32, companies over a 5-year period from 2013 to 2017 to arrive at the numbers below.
The conclusion was that new drugs are unable to cover the costs of failures within the pipeline.
Pharmaceutical companies are struggling with a drug development model where the Internal Rate of Return ((IRR)) on the significant R&D expenditures has been steadily declining. Last year, we had highlighted the issue of low Return on Investment ((ROI)) on R&D budgets. Already believed to be in the low single digits, the IRR runs the risk of heading towards zero and perhaps worse by 2020. These were the insights from a compelling analysis by industry writer Kelvin Stott as well as prior analyses by consulting firms Deloitte and the Boston Consulting Group.
There are many reasons for this decline in IRR, including rising clinical costs, regulatory environment, pressure from insurers, and generic competition. But one of the key reasons is the scarcity of opportunities to create blockbuster drugs, and as Stott explained, the law of diminishing returns which makes it harder for the industry to create the next blockbuster that can surpass the previous one.
Pharmas are being forced to invest in greater innovation to reverse the decline in IRR. The new science around immunotherapies, personalized medicine, gene editing, regenerative medicine, etc., create opportunities for future blockbusters, while the existing money-makers are looking at shrinking revenues as they begin to compete with biosimilars and generics. A pharmaceutical research firm, IQVIA Institute, estimates that over the next 5-year period from 2019 to 2023, brand losses from the expiration of exclusivity will reach $95 billion, well ahead of the $65 billion over the prior 5-year period.
All this is happening at a time where there is also a growing push toward managing drug prices. Declining ROI on R&D budgets, cash cows under threat due to patent protection expiration, and growing pressure on the top line as the clamor for drug price control gets louder, is a difficult conundrum that the industry now faces.
If the cost of drug development is so high, it becomes an unrealistic expectation to regulate drug prices and at the same time not affect product development adversely. The high drug prices are not just an isolated single-factor issue but a more systemic issue that requires a broader solution and not only one that solely targets drug price regulation. Any regulation activity needs to perform a careful balancing act to achieve the twin goals of lower drug prices and strong new product development.
Eventually, M&A Activity Should Benefit
Can M&A be one of the keys for big pharmaceuticals to expand their pipeline of innovative products and in-turn boost the flagging rate of return on product development?
Very much so!
The high total cost of drug development can become an existential question on the health of pharmaceutical companies as we cross over into the next decade. The declining return on in-house R&D will continue to create pressure on the pharmaceutical companies to a point where they are forced to broadly expand their M&A effort with renewed urgency.
Perhaps, 2019 may well become the year when transaction activity begins to ramp higher. And when it does, it cannot be a one-year event but the beginning of a sustained consolidation period recasting the industry dynamics.
Conclusion
The best way to be positioned for rising M&A activity in biotechs will be to have portfolio exposure to the sector. After all, if the M&A pace sustains, then a rising tide will lift all boats. A mix of promising companies across the market cap continuum can have an advantage of even being potential M&A targets. Building out just an acquisition-driven portfolio doesn't work out most of the time. Many potential hot acquisition targets have been waiting for a long time. The SEC filing associated with the December 2018 acquisition of Tesaro (TSRO) by GlaxoSmithKline is an interesting read on the lengthy attempt to auction off the company since mid-2017, finally finding success over 18 months later at a much lower valuation.
The biotech indexes have got off to a strong start this year, after a nerve-wracking ending in 2018 which saw the group amongst the worst performers during the market slide. For the full year 2018, the Nasdaq Biotechnology Index (IBB) was down -10% and the S&P Biotechnology Select Index (XBI) was down -15%. The Prudent Biotech Portfolio was up +27% over the same period. As of this Friday, February 8, the biotech indexes had double-digit gains for the year.
Biotech fortunes can change often and on even a small data readout. So one has to keep refreshing the watch list and biotech exposure in the portfolio. A few promising biotech companies, some of which may be now or in the past part of the Prudent Biotech model portfolios, include Vertex Pharmaceuticals (VRTX), Exelis (EXEL), Regeneron (REGN), Incyte Corporation (INCY), Amarin (AMRN), Intercept Pharmaceuticals (ICPT), Fibrogen (FGEN), Sage Therapeutics (SAGE), Denali Therapeutics (DNLI), Array BioPharma (ARRY), Arena Pharmaceuticals (ARNA), Acadia Pharmaceuticals (ACAD), Blueprint Medicines (BPMC), Codexis (CDXS), Global Blood Therapeutics (GBT), BioCryst Pharmaceuticals (BCRX), Uniqure (QURE), Biohaven Pharmaceutical (BHVN), GW Pharmaceuticals (GWPH), Vericel Corporation (VCEL), Mirati Therapeutics (MRTX), Ra Pharmaceuticals (RARX), Arrowhead Pharmaceuticals (ARWR), and Agenus (AGEN). Sector exposure can also be acquired through heavily diversified ETFs like IBB and S&P Biotechnology Select (XBI). In addition, there are higher-risk, leveraged ETFs for bullish (LABU) and bearish (LABD) outlooks.
Once again, take a portfolio approach in biotechs to overcome mistakes. Next week a hopeful budget resolution and positive updates from the visits to China by trade negotiators can assist stocks in maintaining the uptrend.
Author's note: As always, kindly do your own due diligence.
The article was first published on Seeking Alpha.
The post Biotech Bonanza: The M&A Theme appeared first on Prudent Biotech.
International
Illegal Immigrants Leave US Hospitals With Billions In Unpaid Bills
Illegal Immigrants Leave US Hospitals With Billions In Unpaid Bills
By Autumn Spredemann of The Epoch Times
Tens of thousands of illegal…

By Autumn Spredemann of The Epoch Times
Tens of thousands of illegal immigrants are flooding into U.S. hospitals for treatment and leaving billions in uncompensated health care costs in their wake.
The House Committee on Homeland Security recently released a report illustrating that from the estimated $451 billion in annual costs stemming from the U.S. border crisis, a significant portion is going to health care for illegal immigrants.
With the majority of the illegal immigrant population lacking any kind of medical insurance, hospitals and government welfare programs such as Medicaid are feeling the weight of these unanticipated costs.
Apprehensions of illegal immigrants at the U.S. border have jumped 48 percent since the record in fiscal year 2021 and nearly tripled since fiscal year 2019, according to Customs and Border Protection data.
Last year broke a new record high for illegal border crossings, surpassing more than 3.2 million apprehensions.
And with that sea of humanity comes the need for health care and, in most cases, the inability to pay for it.
In January, CEO of Denver Health Donna Lynne told reporters that 8,000 illegal immigrants made roughly 20,000 visits to the city’s health system in 2023.
The total bill for uncompensated care costs last year to the system totaled $140 million, said Dane Roper, public information officer for Denver Health. More than $10 million of it was attributed to “care for new immigrants,” he told The Epoch Times.
Though the amount of debt assigned to illegal immigrants is a fraction of the total, uncompensated care costs in the Denver Health system have risen dramatically over the past few years.
The total uncompensated costs in 2020 came to $60 million, Mr. Roper said. In 2022, the number doubled, hitting $120 million.
He also said their city hospitals are treating issues such as “respiratory illnesses, GI [gastro-intenstinal] illnesses, dental disease, and some common chronic illnesses such as asthma and diabetes.”
“The perspective we’ve been trying to emphasize all along is that providing healthcare services for an influx of new immigrants who are unable to pay for their care is adding additional strain to an already significant uncompensated care burden,” Mr. Roper said.
He added this is why a local, state, and federal response to the needs of the new illegal immigrant population is “so important.”
Colorado is far from the only state struggling with a trail of unpaid hospital bills.

Dr. Robert Trenschel, CEO of the Yuma Regional Medical Center situated on the Arizona–Mexico border, said on average, illegal immigrants cost up to three times more in human resources to resolve their cases and provide a safe discharge.
“Some [illegal] migrants come with minor ailments, but many of them come in with significant disease,” Dr. Trenschel said during a congressional hearing last year.
“We’ve had migrant patients on dialysis, cardiac catheterization, and in need of heart surgery. Many are very sick.”
He said many illegal immigrants who enter the country and need medical assistance end up staying in the ICU ward for 60 days or more.
A large portion of the patients are pregnant women who’ve had little to no prenatal treatment. This has resulted in an increase in babies being born that require neonatal care for 30 days or longer.
Dr. Trenschel told The Epoch Times last year that illegal immigrants were overrunning healthcare services in his town, leaving the hospital with $26 million in unpaid medical bills in just 12 months.
ER Duty to Care
The Emergency Medical Treatment and Labor Act of 1986 requires that public hospitals participating in Medicare “must medically screen all persons seeking emergency care … regardless of payment method or insurance status.”
The numbers are difficult to gauge as the policy position of the Centers for Medicare & Medicaid Services (CMS) is that it “will not require hospital staff to ask patients directly about their citizenship or immigration status.”
In southern California, again close to the border with Mexico, some hospitals are struggling with an influx of illegal immigrants.
American patients are enduring longer wait times for doctor appointments due to a nursing shortage in the state, two health care professionals told The Epoch Times in January.
A health care worker at a hospital in Southern California, who asked not to be named for fear of losing her job, told The Epoch Times that “the entire health care system is just being bombarded” by a steady stream of illegal immigrants.
“Our healthcare system is so overwhelmed, and then add on top of that tuberculosis, COVID-19, and other diseases from all over the world,” she said.

A newly-enacted law in California provides free healthcare for all illegal immigrants residing in the state. The law could cost taxpayers between $3 billion and $6 billion per year, according to recent estimates by state and federal lawmakers.
In New York, where the illegal immigration crisis has manifested most notably beyond the southern border, city and state officials have long been accommodating of illegal immigrants’ healthcare costs.
Since June 2014, when then-mayor Bill de Blasio set up The Task Force on Immigrant Health Care Access, New York City has worked to expand avenues for illegal immigrants to get free health care.
“New York City has a moral duty to ensure that all its residents have meaningful access to needed health care, regardless of their immigration status or ability to pay,” Mr. de Blasio stated in a 2015 report.
The report notes that in 2013, nearly 64 percent of illegal immigrants were uninsured. Since then, tens of thousands of illegal immigrants have settled in the city.
“The uninsured rate for undocumented immigrants is more than three times that of other noncitizens in New York City (20 percent) and more than six times greater than the uninsured rate for the rest of the city (10 percent),” the report states.
The report states that because healthcare providers don’t ask patients about documentation status, the task force lacks “data specific to undocumented patients.”
Some health care providers say a big part of the issue is that without a clear path to insurance or payment for non-emergency services, illegal immigrants are going to the hospital due to a lack of options.
“It’s insane, and it has been for years at this point,” Dana, a Texas emergency room nurse who asked to have her full name omitted, told The Epoch Times.
Working for a major hospital system in the greater Houston area, Dana has seen “a zillion” migrants pass through under her watch with “no end in sight.” She said many who are illegal immigrants arrive with treatable illnesses that require simple antibiotics. “Not a lot of GPs [general practitioners] will see you if you can’t pay and don’t have insurance.”
She said the “undocumented crowd” tends to arrive with a lot of the same conditions. Many find their way to Houston not long after crossing the southern border. Some of the common health issues Dana encounters include dehydration, unhealed fractures, respiratory illnesses, stomach ailments, and pregnancy-related concerns.
“This isn’t a new problem, it’s just worse now,” Dana said.

Medicaid Factor
One of the main government healthcare resources illegal immigrants use is Medicaid.
All those who don’t qualify for regular Medicaid are eligible for Emergency Medicaid, regardless of immigration status. By doing this, the program helps pay for the cost of uncompensated care bills at qualifying hospitals.
However, some loopholes allow access to the regular Medicaid benefits. “Qualified noncitizens” who haven’t been granted legal status within five years still qualify if they’re listed as a refugee, an asylum seeker, or a Cuban or Haitian national.
Yet the lion’s share of Medicaid usage by illegal immigrants still comes through state-level benefits and emergency medical treatment.
A Congressional report highlighted data from the CMS, which showed total Medicaid costs for “emergency services for undocumented aliens” in fiscal year 2021 surpassed $7 billion, and totaled more than $5 billion in fiscal 2022.
Both years represent a significant spike from the $3 billion in fiscal 2020.
An employee working with Medicaid who asked to be referred to only as Jennifer out of concern for her job, told The Epoch Times that at a state level, it’s easy for an illegal immigrant to access the program benefits.
Jennifer said that when exceptions are sent from states to CMS for approval, “denial is actually super rare. It’s usually always approved.”
She also said it comes as no surprise that many of the states with the highest amount of Medicaid spending are sanctuary states, which tend to have policies and laws that shield illegal immigrants from federal immigration authorities.
Moreover, Jennifer said there are ways for states to get around CMS guidelines. “It’s not easy, but it can and has been done.”
The first generation of illegal immigrants who arrive to the United States tend to be healthy enough to pass any pre-screenings, but Jennifer has observed that the subsequent generations tend to be sicker and require more access to care. If a family is illegally present, they tend to use Emergency Medicaid or nothing at all.
The Epoch Times asked Medicaid Services to provide the most recent data for the total uncompensated care that hospitals have reported. The agency didn’t respond.
Continue reading over at The Epoch Times
Uncategorized
Fast-food chain closes restaurants after Chapter 11 bankruptcy
Several major fast-food chains recently have struggled to keep restaurants open.

Competition in the fast-food space has been brutal as operators deal with inflation, consumers who are worried about the economy and their jobs and, in recent months, the falling cost of eating at home.
Add in that many fast-food chains took on more debt during the covid pandemic and that labor costs are rising, and you have a perfect storm of problems.
It's a situation where Restaurant Brands International (QSR) has suffered as much as any company.
Related: Wendy's menu drops a fan favorite item, adds something new
Three major Burger King franchise operators filed for bankruptcy in 2023, and the chain saw hundreds of stores close. It also saw multiple Popeyes franchisees move into bankruptcy, with dozens of locations closing.
RBI also stepped in and purchased one of its key franchisees.
"Carrols is the largest Burger King franchisee in the United States today, operating 1,022 Burger King restaurants in 23 states that generated approximately $1.8 billion of system sales during the 12 months ended Sept. 30, 2023," RBI said in a news release. Carrols also owns and operates 60 Popeyes restaurants in six states."
The multichain company made the move after two of its large franchisees, Premier Kings and Meridian, saw multiple locations not purchased when they reached auction after Chapter 11 bankruptcy filings. In that case, RBI bought select locations but allowed others to close.

Image source: Chen Jianli/Xinhua via Getty
Another fast-food chain faces bankruptcy problems
Bojangles may not be as big a name as Burger King or Popeye's, but it's a popular chain with more than 800 restaurants in eight states.
"Bojangles is a Carolina-born restaurant chain specializing in craveable Southern chicken, biscuits and tea made fresh daily from real recipes, and with a friendly smile," the chain says on its website. "Founded in 1977 as a single location in Charlotte, our beloved brand continues to grow nationwide."
Like RBI, Bojangles uses a franchise model, which makes it dependent on the financial health of its operators. The company ultimately saw all its Maryland locations close due to the financial situation of one of its franchisees.
Unlike. RBI, Bojangles is not public — it was taken private by Durational Capital Management LP and Jordan Co. in 2018 — which means the company does not disclose its financial information to the public.
That makes it hard to know whether overall softness for the brand contributed to the chain seeing its five Maryland locations after a Chapter 11 bankruptcy filing.
Bojangles has a messy bankruptcy situation
Even though the locations still appear on the Bojangles website, they have been shuttered since late 2023. The locations were operated by Salim Kakakhail and Yavir Akbar Durranni. The partners operated under a variety of LLCs, including ABS Network, according to local news channel WUSA9.
The station reported that the owners face a state investigation over complaints of wage theft and fraudulent W2s. In November Durranni and ABS Network filed for bankruptcy in New Jersey, WUSA9 reported.
"Not only do former employees say these men owe them money, WUSA9 learned the former owners owe the state, too, and have over $69,000 in back property taxes."
Former employees also say that the restaurant would regularly purchase fried chicken from Popeyes and Safeway when it ran out in their stores, the station reported.
Bojangles sent the station a comment on the situation.
"The franchisee is no longer in the Bojangles system," the company said. "However, it is important to note in your coverage that franchisees are independent business owners who are licensed to operate a brand but have autonomy over many aspects of their business, including hiring employees and payroll responsibilities."
Kakakhail and Durranni did not respond to multiple requests for comment from WUSA9.
bankruptcy pandemicUncategorized
Industrial Production Increased 0.1% in February
From the Fed: Industrial Production and Capacity Utilization
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 p…
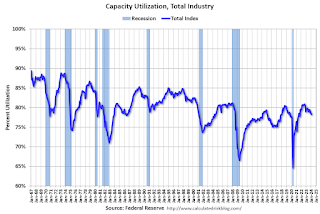
Industrial production edged up 0.1 percent in February after declining 0.5 percent in January. In February, the output of manufacturing rose 0.8 percent and the index for mining climbed 2.2 percent. Both gains partly reflected recoveries from weather-related declines in January. The index for utilities fell 7.5 percent in February because of warmer-than-typical temperatures. At 102.3 percent of its 2017 average, total industrial production in February was 0.2 percent below its year-earlier level. Capacity utilization for the industrial sector remained at 78.3 percent in February, a rate that is 1.3 percentage points below its long-run (1972–2023) average.Click on graph for larger image.
emphasis added
This graph shows Capacity Utilization. This series is up from the record low set in April 2020, and above the level in February 2020 (pre-pandemic).
Capacity utilization at 78.3% is 1.3% below the average from 1972 to 2022. This was below consensus expectations.
Note: y-axis doesn't start at zero to better show the change.
 The second graph shows industrial production since 1967.
The second graph shows industrial production since 1967.Industrial production increased to 102.3. This is above the pre-pandemic level.
Industrial production was above consensus expectations.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International1 week ago
International1 week agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 week ago
International1 week agoWalmart launches clever answer to Target’s new membership program
-

 Spread & Containment2 days ago
Spread & Containment2 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
































