Funding: Medical Research Future Fund, mRNA Victoria, Jack Ma Foundation, IFM Investors, Australian Research Council and National Health and Medical Research Council
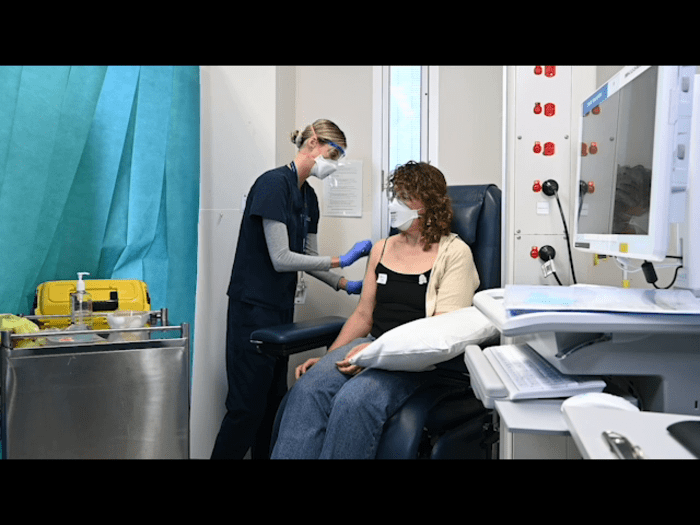
The first six participants in the clinical trial of two Melbourne-made COVID-19 vaccines have been safely administered their doses, a significant moment in the development of the new vaccine candidates.
The two vaccine candidates were created by researchers at the Peter Doherty Institute for Infection and Immunity (Doherty Institute) and Monash Institute of Pharmaceutical Sciences (MIPS) and are distinct from existing vaccines in use around the globe.
These vaccines focus the immune response on the tip of the SARS-CoV-2 spike protein, called the receptor binding domain (RBD). The RBD enables the virus to enter and infect cells in the body and elicits over 90 per cent of neutralising antibodies (antibodies that can block the virus) following SARS-CoV-2 infection.
The two candidates are:
- Doherty RBD protein vaccine – uses part of the virus protein, rather than genetic material or another virus, to elicit an immune response.
- MIPS RBD mRNA vaccine – represents the virus genetic sequence that codes for the tip of the spike, which will lead to production of the RBD protein.
In addition, they are ‘proof-of-principle’ variant vaccines that present the Beta variant to the immune system, which was of the greatest concern when these vaccines were designed. Furthermore, the Beta variant has two of the same key RBD mutations as the Omicron variants (BA.1 and BA.2), so they may also improve immunity to Omicron.
The randomised, double-blind, placebo-controlled study is the first time a side-by-side comparison will be undertaken of protein and mRNA based COVID-19 vaccine platforms, each vaccine at three dose levels (lower, intermediate and higher doses).
It will assess the safety and efficacy of a single dose of these vaccines as a fourth dose of a COVID-19 vaccine, with all participants already having received their third dose of an existing vaccine at least three months prior.
These first six participants were assessed at the Royal Melbourne Hospital and then randomised to receive either the RBD protein vaccine, RBD mRNA vaccine or a placebo.
Principal Investigator, University of Melbourne Professor Terry Nolan, Head of the Vaccine and Immunisation Research Group at the Doherty Institute, said that the participants will be required to keep a diary of any symptoms they may experience over the following month.
“In addition, these initial participants and all others in the study will have numerous assessments, including a blood test, after 30 days so we can analyse their antibody response,” Professor Nolan said.
“Only after all 114 participants have been vaccinated and provided a blood sample 30 days post-vaccination will we receive the data and be able to begin analysing how the vaccines have performed.”
Professor Colin Pouton of MIPS, who led the development of the RBD mRNA vaccine, said it has been an exciting moment for the team to see patients receive the first doses.
“It’s been incredible to see the potential of mRNA vaccines over the last two years, and we’re excited to see Australia’s first mRNA vaccine reach this milestone,” said Professor Pouton.
“Getting to this stage has been an enormous team effort across both institutes, and we look forward to seeing how the vaccines perform in clinic.”
University of Melbourne Professor Sharon Lewin, Director of the Doherty Institute, said next generation vaccines will be an important tool to combat new variants as they arise and to help bring the global pandemic under control.
“There is still a need for millions of doses to be administered and it is important that Australia has the ability to manufacture its own vaccines to protect not only our communities, but those in low- and middle-income countries who have yet to receive protection,” said Professor Lewin.
“The unique thing about both these vaccines is their ability to be rapidly modified to target distinct RBD mutations which may arise in future variants.
Monash University Professor Chris Porter, Director of MIPS, said reaching this milestone is a true testament to the collaborative spirit of the trial.
“This trial is quite unique in that it’s the first time a side-by-side comparison will be undertaken of two new COVID-19 platforms. That this is possible within a single trial is a testament to the collaborative ethos of the research teams at MIPS and the Doherty Institute and the broad support of the Australian and Victorian governments and our industry partners,” said Professor Porter.
It is uncertain how long the trial will take to complete.
“It is virtually impossible to predict how long this will take as a number of human factors can cause delays,” explained Professor Nolan.
Volunteers are still being recruited and the trial is open to any healthy individual aged 18-64 living in Victoria, who has received their third dose of a COVID-19 vaccine at least three months ago.
People who have been infected with COVID-19 are also eligible provided they had their infection at least three months prior and have had their third vaccine dose.
Those interested in participating in the clinical trial are encouraged to visit doherty.edu.au to find out more, or email virgo-studies@unimelb.edu.au or call 8344 9325.
The Doherty Institute and MIPS would like to thank their funders and other supporting organisations including the University of Melbourne, The Royal Melbourne Hospital, CSIRO, Seqirus and IDT Australia.
Ends
Method of Research
Randomized controlled/clinical trial
Subject of Research
People