International
Top Penny Stocks to Add to Your Watchlist Right Now
Can these penny stocks push up in January 2022?
The post Top Penny Stocks to Add to Your Watchlist Right Now appeared first on Penny Stocks to Buy, Picks, News and Information | PennyStocks.com.
3 Top Penny Stocks to Watch As We Turn the Corner in January
With such a large range of penny stocks to choose from, finding the best ones for your watchlist can be challenging. But, with the right amount of research and a dedicated trading strategy, finding the best penny stocks to buy can be much easier than previously imagined. To find penny stocks that could be worth it, it takes a thorough understanding of what is going on in the stock market and how to trade.
When it comes to trading penny stocks, there are two main strategies that investors utilize. On one hand, we have long-term trading. While this is less common given the sheer amount of volatility that penny stocks see, it is a viable strategy. On the other hand, we have short term or swing trading. This tends to be the most popular trading method solely because penny stocks move frequently.
[Read More] Best Penny Stocks To Buy Now? 3 To Watch After Trump Stocks Surge
While no one strategy is better than another, it all comes down to what type of trader you are. So, knowing what type of trader you are is paramount to making money with penny stocks in 2022. Considering all of this, let’s take a look at three penny stocks to watch right now.
3 Penny Stocks to Add to Your Watchlist in January
- Borqs Technologies Inc. (NASDAQ: BRQS)
- Ambev ADR (NYSE: ABEV)
- Ideanomics Inc. (NASDAQ: IDEX)
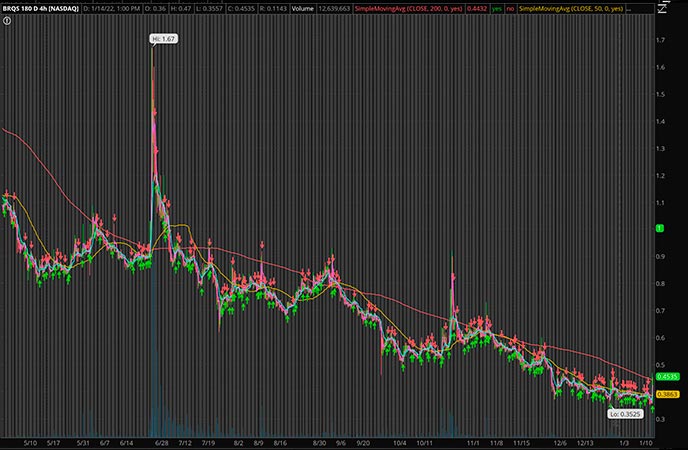
Borqs Technologies Inc. (NASDAQ: BRQS)
One of the bigger gainers of the day so far is BRQS stock. By EOD, shares of BRQS stock had shot up by over 20% to north of $0.44 per share. While many large gains occur without news, only a few weeks ago, Borqs made an exciting announcement. The company stated that it has entered into the EV smart cockpit market.
“We are very pleased to team up with Cheyin to leverage our deep expertise in the IoT and embedded software solutions. Borqs has developed software for In-Vehicle-Infotainment systems in the past several years which were installed in some of the China-made automobiles. Digital transformation in the automobile industry is accelerating in the coming years because of the robust growth of electric vehicles.”
Pat Chan, the CEO of Borqs Technologies
If you’re not familiar, Borqs is a Qualcomm portfolio company, and is a large player in the IoT, products, and software market. The company has a large range of IP as well as software that it offers to its customers. Recently, Borqs has also moved into the 5G market through new products for phones and hotspots. Right now, there is a major amount of emphasis on tech penny stocks. And, with this new update putting Borqs into the EV market, it’s clear the company could remain in the public eye. With that in mind, will it be on your penny stocks watchlist?
Ambev ADR (NYSE: ABEV)
ABEV is a penny stock that we’ve covered numerous times over the past few weeks. And again today, shares of the beverage company are climbing during trading. In the past five days, ABEV stock has shot up by over 7%. While shares are still down for the year period, it looks like we are witnessing a small bullish turnaround with Ambev right now.
So, why the bullish sentiment with Ambev ADR in January? Well, since its earnings report for the third quarter came out a few months ago, investors have showed a renewed confidence in the future of the company. It stated that in its keys markets, it was able to grow in volume by around 8%.
[Read More] 4 Top Penny Stocks To Watch For A Short Squeeze
One of the interesting aspects of Ambev or any beverage company is that they tend to see sales increase in both positive and negative economic times. For example, when people were at home due to the pandemic, the sale of alcohol rose significantly. And now that people are once again going out, we’ve seen those same sales continue to rise. While ABEV stock can be quite volatile, it could also be worth looking into for your list of penny stocks to watch.

Ideanomics Inc. (NASDAQ: IDEX)
Another popular penny stock to watch right now is Ideanomics Inc. Today, shares of IDEX managed to climb by almost 5%, ending the day at just under $1.20 per share. If you’re not familiar, Ideanomics operates under two main categories. On one hand, its Ideanomics Mobility division invests in budding EV technology. On the other hand, its Ideanomics Capital division does the same but with new Fintech related offerings. The most recent news from IDEX came as the company announced an investment into InoBat. This is a company working on the development of new electric batteries.
“InoBat prides itself on providing innovative solutions across the entire battery value chain thanks to our own ‘cradle-to-cradle’ approach. We are thrilled to join hands with Ideanomics, a like-minded company with vehicles across a wide range of industries.”
The CEO of InoBat, Marian Bocek
With the major bullish emphasis on EV penny stocks right now, it’s clear that IDEX is in focus. Whether this makes it worth adding to your watchlist however is up to you. But, investors should continue to consider the future of both industries that Ideanomics works in before making a decision.

Which Penny Stocks Are You Watching Right Now?
If you’re looking for the best penny stocks to buy, there are hundreds of options to choose from. But, investors need to have a consistent trading strategy on hand and a thorough understanding of how to trade in order to have the best chance of making money with penny stocks. Right now, there are quite a few industries that investors are interested in. This includes biotech penny stocks, tech penny stocks, and in the near future, reopening penny stocks.
[Read More] Stock Market News For Today, January 14th, 2022
Within each of these industries are penny stocks that could be worth it; but it depends on specific companies and their fundamentals. At the end of the day, trading is a very individual task, and should be approached as such. With all of that in mind, which penny stocks are you watching right now?
If you enjoyed this article and you’re interested in learning how to trade so you can have the best chance to profit consistently then you need to checkout this YouTube channel. CLICK HERE RIGHT NOW!
The post Top Penny Stocks to Add to Your Watchlist Right Now appeared first on Penny Stocks to Buy, Picks, News and Information | PennyStocks.com.
reopening pandemic nasdaq stocks penny stocks trump chinaInternational
Congress’ failure so far to deliver on promise of tens of billions in new research spending threatens America’s long-term economic competitiveness
A deal that avoided a shutdown also slashed spending for the National Science Foundation, putting it billions below a congressional target intended to…

Federal spending on fundamental scientific research is pivotal to America’s long-term economic competitiveness and growth. But less than two years after agreeing the U.S. needed to invest tens of billions of dollars more in basic research than it had been, Congress is already seriously scaling back its plans.
A package of funding bills recently passed by Congress and signed by President Joe Biden on March 9, 2024, cuts the current fiscal year budget for the National Science Foundation, America’s premier basic science research agency, by over 8% relative to last year. That puts the NSF’s current allocation US$6.6 billion below targets Congress set in 2022.
And the president’s budget blueprint for the next fiscal year, released on March 11, doesn’t look much better. Even assuming his request for the NSF is fully funded, it would still, based on my calculations, leave the agency a total of $15 billion behind the plan Congress laid out to help the U.S. keep up with countries such as China that are rapidly increasing their science budgets.
I am a sociologist who studies how research universities contribute to the public good. I’m also the executive director of the Institute for Research on Innovation and Science, a national university consortium whose members share data that helps us understand, explain and work to amplify those benefits.
Our data shows how underfunding basic research, especially in high-priority areas, poses a real threat to the United States’ role as a leader in critical technology areas, forestalls innovation and makes it harder to recruit the skilled workers that high-tech companies need to succeed.
A promised investment
Less than two years ago, in August 2022, university researchers like me had reason to celebrate.
Congress had just passed the bipartisan CHIPS and Science Act. The science part of the law promised one of the biggest federal investments in the National Science Foundation in its 74-year history.
The CHIPS act authorized US$81 billion for the agency, promised to double its budget by 2027 and directed it to “address societal, national, and geostrategic challenges for the benefit of all Americans” by investing in research.
But there was one very big snag. The money still has to be appropriated by Congress every year. Lawmakers haven’t been good at doing that recently. As lawmakers struggle to keep the lights on, fundamental research is quickly becoming a casualty of political dysfunction.
Research’s critical impact
That’s bad because fundamental research matters in more ways than you might expect.
For instance, the basic discoveries that made the COVID-19 vaccine possible stretch back to the early 1960s. Such research investments contribute to the health, wealth and well-being of society, support jobs and regional economies and are vital to the U.S. economy and national security.
Lagging research investment will hurt U.S. leadership in critical technologies such as artificial intelligence, advanced communications, clean energy and biotechnology. Less support means less new research work gets done, fewer new researchers are trained and important new discoveries are made elsewhere.
But disrupting federal research funding also directly affects people’s jobs, lives and the economy.
Businesses nationwide thrive by selling the goods and services – everything from pipettes and biological specimens to notebooks and plane tickets – that are necessary for research. Those vendors include high-tech startups, manufacturers, contractors and even Main Street businesses like your local hardware store. They employ your neighbors and friends and contribute to the economic health of your hometown and the nation.
Nearly a third of the $10 billion in federal research funds that 26 of the universities in our consortium used in 2022 directly supported U.S. employers, including:
A Detroit welding shop that sells gases many labs use in experiments funded by the National Institutes of Health, National Science Foundation, Department of Defense and Department of Energy.
A Dallas-based construction company that is building an advanced vaccine and drug development facility paid for by the Department of Health and Human Services.
More than a dozen Utah businesses, including surveyors, engineers and construction and trucking companies, working on a Department of Energy project to develop breakthroughs in geothermal energy.
When Congress shortchanges basic research, it also damages businesses like these and people you might not usually associate with academic science and engineering. Construction and manufacturing companies earn more than $2 billion each year from federally funded research done by our consortium’s members.

Jobs and innovation
Disrupting or decreasing research funding also slows the flow of STEM – science, technology, engineering and math – talent from universities to American businesses. Highly trained people are essential to corporate innovation and to U.S. leadership in key fields, such as AI, where companies depend on hiring to secure research expertise.
In 2022, federal research grants paid wages for about 122,500 people at universities that shared data with my institute. More than half of them were students or trainees. Our data shows that they go on to many types of jobs but are particularly important for leading tech companies such as Google, Amazon, Apple, Facebook and Intel.
That same data lets me estimate that over 300,000 people who worked at U.S. universities in 2022 were paid by federal research funds. Threats to federal research investments put academic jobs at risk. They also hurt private sector innovation because even the most successful companies need to hire people with expert research skills. Most people learn those skills by working on university research projects, and most of those projects are federally funded.
High stakes
If Congress doesn’t move to fund fundamental science research to meet CHIPS and Science Act targets – and make up for the $11.6 billion it’s already behind schedule – the long-term consequences for American competitiveness could be serious.
Over time, companies would see fewer skilled job candidates, and academic and corporate researchers would produce fewer discoveries. Fewer high-tech startups would mean slower economic growth. America would become less competitive in the age of AI. This would turn one of the fears that led lawmakers to pass the CHIPS and Science Act into a reality.
Ultimately, it’s up to lawmakers to decide whether to fulfill their promise to invest more in the research that supports jobs across the economy and in American innovation, competitiveness and economic growth. So far, that promise is looking pretty fragile.
This is an updated version of an article originally published on Jan. 16, 2024.
Jason Owen-Smith receives research support from the National Science Foundation, the National Institutes of Health, the Alfred P. Sloan Foundation and Wellcome Leap.
economic growth covid-19 grants congress vaccine chinaInternational
What’s Driving Industrial Development in the Southwest U.S.
The post-COVID-19 pandemic pipeline, supply imbalances, investment and construction challenges: these are just a few of the topics address by a powerhouse…

The post-COVID-19 pandemic pipeline, supply imbalances, investment and construction challenges: these are just a few of the topics address by a powerhouse panel of executives in industrial real estate this week at NAIOP’s I.CON West in Long Beach, California. Led by Dawn McCombs, principal and Denver lead industrial specialist for Avison Young, the panel tackled some of the biggest issues facing the sector in the Western U.S.
Starting with the pandemic in 2020 and continuing through 2022, McCombs said, the industrial sector experienced a huge surge in demand, resulting in historic vacancies, rent growth and record deliveries. Operating fundamentals began to normalize in 2023 and construction starts declined, certainly impacting vacancy and absorption moving forward.
“Development starts dropped by 65% year-over-year across the U.S. last year. In Q4, we were down 25% from pre-COVID norms,” began Megan Creecy-Herman, president, U.S. West Region, Prologis, noting that all of that is setting us up to see an improvement of fundamentals in the market. “U.S. vacancy ended 2023 at about 5%, which is very healthy.”
Vacancies are expected to grow in Q1 and Q2, peaking mid-year at around 7%. Creecy-Herman expects to see an increase in absorption as customers begin to have confidence in the economy, and everyone gets some certainty on what the Fed does with interest rates.
“It’s an interesting dynamic to see such a great increase in rents, which have almost doubled in some markets,” said Reon Roski, CEO, Majestic Realty Co. “It’s healthy to see a slowing down… before [rents] go back up.”
Pre-pandemic, a lot of markets were used to 4-5% vacancy, said Brooke Birtcher Gustafson, fifth-generation president of Birtcher Development. “Everyone was a little tepid about where things are headed with a mediocre outlook for 2024, but much of this is normalizing in the Southwest markets.”
McCombs asked the panel where their companies found themselves in the construction pipeline when the Fed raised rates in 2022.
In Salt Lake City, said Angela Eldredge, chief operations officer at Price Real Estate, there is a typical 12-18-month lead time on construction materials. “As rates started to rise in 2022, lots of permits had already been pulled and construction starts were beginning, so those project deliveries were in fall 2023. [The slowdown] was good for our market because it kept rates high, vacancies lower and helped normalize the market to a healthy pace.”
A supply imbalance can stress any market, and Gustafson joked that the current imbalance reminded her of a favorite quote from the movie Super Troopers: “Desperation is a stinky cologne.” “We’re all still a little crazed where this imbalance has put us, but for the patient investor and owner, there will be a rebalancing and opportunity for the good quality real estate to pass the sniff test,” she said.
At Bircher, Gustafson said that mid-pandemic, there were predictions that one billion square feet of new product would be required to meet tenant demand, e-commerce growth and safety stock. That transition opened a great opportunity for investors to run at the goal. “In California, the entitlement process is lengthy, around 24-36 months to get from the start of an acquisition to the completion of a building,” she said. Fast forward to 2023-2024, a lot of what is being delivered in 2024 is the result of that chase.
“Being an optimistic developer, there is good news. The supply imbalance helped normalize what was an unsustainable surge in rents and land values,” she said. “It allowed corporate heads of real estate to proactively evaluate growth opportunities, opened the door for contrarian investors to land bank as values drop, and provided tenants with options as there is more product. Investment goals and strategies have shifted, and that’s created opportunity for buyers.”
“Developers only know how to run and develop as much as we can,” said Roski. “There are certain times in cycles that we are forced to slow down, which is a good thing. In the last few years, Majestic has delivered 12-14 million square feet, and this year we are developing 6-8 million square feet. It’s all part of the cycle.”
Creecy-Herman noted that compared to the other asset classes and opportunities out there, including office and multifamily, industrial remains much more attractive for investment. “That was absolutely one of the things that underpinned the amount of investment we saw in a relatively short time period,” she said.
Market rent growth across Los Angeles, Inland Empire and Orange County moved up more than 100% in a 24-month period. That created opportunities for landlords to flexible as they’re filling up their buildings. “Normalizing can be uncomfortable especially after that kind of historic high, but at the same time it’s setting us up for strong years ahead,” she said.
Issues that owners and landlords are facing with not as much movement in the market is driving a change in strategy, noted Gustafson. “Comps are all over the place,” she said. “You have to dive deep into every single deal that is done to understand it and how investment strategies are changing.”
Tenants experienced a variety of challenges in the pandemic years, from supply chain to labor shortages on the negative side, to increased demand for products on the positive, McCombs noted.
“Prologis has about 6,700 customers around the world, from small to large, and the universal lesson [from the pandemic] is taking a more conservative posture on inventories,” Creecy-Herman said. “Customers are beefing up inventories, and that conservatism in the supply chain is a lesson learned that’s going to stick with us for a long time.” She noted that the company has plenty of clients who want to take more space but are waiting on more certainty from the broader economy.
“E-commerce grew by 8% last year, and we think that’s going to accelerate to 10% this year. This is still less than 25% of all retail sales, so the acceleration we’re going to see in e-commerce… is going to drive the business forward for a long time,” she said.
Roski noted that customers continually re-evaluate their warehouse locations, expanding during the pandemic and now consolidating but staying within one delivery day of vast consumer bases.
“This is a generational change,” said Creecy-Herman. “Millions of young consumers have one-day delivery as a baseline for their shopping experience. Think of what this means for our business long term to help our customers meet these expectations.”
McCombs asked the panelists what kind of leasing activity they are experiencing as a return to normalcy is expected in 2024.
“During the pandemic, shifts in the ports and supply chain created a build up along the Mexican border,” said Roski, noting border towns’ importance to increased manufacturing in Mexico. A shift of populations out of California and into Arizona, Nevada, Texas and Florida have resulted in an expansion of warehouses in those markets.
Eldridge said that Salt Lake City’s “sweet spot” is 100-200 million square feet, noting that the market is best described as a mid-box distribution hub that is close to California and Midwest markets. “Our location opens up the entire U.S. to our market, and it’s continuing to grow,” she said.
The recent supply chain and West Coast port clogs prompted significant investment in nearshoring and port improvements. “Ports are always changing,” said Roski, listing a looming strike at East Coast ports, challenges with pirates in the Suez Canal, and water issues in the Panama Canal. “Companies used to fix on one port and that’s where they’d bring in their imports, but now see they need to be [bring product] in a couple of places.”
“Laredo, [Texas,] is one of the largest ports in the U.S., and there’s no water. It’s trucks coming across the border. Companies have learned to be nimble and not focused on one area,” she said.
“All of the markets in the southwest are becoming more interconnected and interdependent than they were previously,” Creecy-Herman said. “In Southern California, there are 10 markets within 500 miles with over 25 million consumers who spend, on average, 10% more than typical U.S. consumers.” Combined with the port complex, those fundamentals aren’t changing. Creecy-Herman noted that it’s less of a California exodus than it is a complementary strategy where customers are taking space in other markets as they grow. In the last 10 years, she noted there has been significant maturation of markets such as Las Vegas and Phoenix. As they’ve become more diversified, customers want to have a presence there.
In the last decade, Gustafson said, the consumer base has shifted. Tenants continue to change strategies to adapt, such as hub-and-spoke approaches. From an investment perspective, she said that strategies change weekly in response to market dynamics that are unprecedented.
McCombs said that construction challenges and utility constraints have been compounded by increased demand for water and power.
“Those are big issues from the beginning when we’re deciding on whether to buy the dirt, and another decision during construction,” Roski said. “In some markets, we order transformers more than a year before they are needed. Otherwise, the time comes [to use them] and we can’t get them. It’s a new dynamic of how leases are structured because it’s something that’s out of our control.” She noted that it’s becoming a bigger issue with electrification of cars, trucks and real estate, and the U.S. power grid is not prepared to handle it.
Salt Lake City’s land constraints play a role in site selection, said Eldridge. “Land values of areas near water are skyrocketing.”
The panelists agreed that a favorable outlook is ahead for 2024, and today’s rebalancing will drive a healthy industry in the future as demand and rates return to normalized levels, creating opportunities for investors, developers and tenants.
This post is brought to you by JLL, the social media and conference blog sponsor of NAIOP’s I.CON West 2024. Learn more about JLL at www.us.jll.com or www.jll.ca.
fed pandemic covid-19 real estate interest rates mexicoInternational
Analyst reviews Apple stock price target amid challenges
Here’s what could happen to Apple shares next.

They said it was bound to happen.
It was Jan. 11, 2024 when software giant Microsoft (MSFT) briefly passed Apple (AAPL) as the most valuable company in the world.
Microsoft's stock closed 0.5% higher, giving it a market valuation of $2.859 trillion.
It rose as much as 2% during the session and the company was briefly worth $2.903 trillion. Apple closed 0.3% lower, giving the company a market capitalization of $2.886 trillion.
"It was inevitable that Microsoft would overtake Apple since Microsoft is growing faster and has more to benefit from the generative AI revolution," D.A. Davidson analyst Gil Luria said at the time, according to Reuters.
The two tech titans have jostled for top spot over the years and Microsoft was ahead at last check, with a market cap of $3.085 trillion, compared with Apple's value of $2.684 trillion.
Analysts noted that Apple had been dealing with weakening demand, including for the iPhone, the company’s main source of revenue.
Demand in China, a major market, has slumped as the country's economy makes a slow recovery from the pandemic and competition from Huawei.
Sales in China of Apple's iPhone fell by 24% in the first six weeks of 2024 compared with a year earlier, according to research firm Counterpoint, as the company contended with stiff competition from a resurgent Huawei "while getting squeezed in the middle on aggressive pricing from the likes of OPPO, vivo and Xiaomi," said senior Analyst Mengmeng Zhang.
“Although the iPhone 15 is a great device, it has no significant upgrades from the previous version, so consumers feel fine holding on to the older-generation iPhones for now," he said.
Big plans for China
Counterpoint said that the first six weeks of 2023 saw abnormally high numbers with significant unit sales being deferred from December 2022 due to production issues.
Apple is planning to open its eighth store in Shanghai – and its 47th across China – on March 21.
Related: Tech News Now: OpenAI says Musk contract 'never existed', Xiaomi's EV, and more
The company also plans to expand its research centre in Shanghai to support all of its product lines and open a new lab in southern tech hub Shenzhen later this year, according to the South China Morning Post.
Meanwhile, over in Europe, Apple announced changes to comply with the European Union's Digital Markets Act (DMA), which went into effect last week, Reuters reported on March 12.
Beginning this spring, software developers operating in Europe will be able to distribute apps to EU customers directly from their own websites instead of through the App Store.
"To reflect the DMA’s changes, users in the EU can install apps from alternative app marketplaces in iOS 17.4 and later," Apple said on its website, referring to the software platform that runs iPhones and iPads.
"Users will be able to download an alternative marketplace app from the marketplace developer’s website," the company said.
Apple has also said it will appeal a $2 billion EU antitrust fine for thwarting competition from Spotify (SPOT) and other music streaming rivals via restrictions on the App Store.
The company's shares have suffered amid all this upheaval, but some analysts still see good things in Apple's future.
Bank of America Securities confirmed its positive stance on Apple, maintaining a buy rating with a steady price target of $225, according to Investing.com.
The firm's analysis highlighted Apple's pricing strategy evolution since the introduction of the first iPhone in 2007, with initial prices set at $499 for the 4GB model and $599 for the 8GB model.
BofA said that Apple has consistently launched new iPhone models, including the Pro/Pro Max versions, to target the premium market.
Analyst says Apple selloff 'overdone'
Concurrently, prices for previous models are typically reduced by about $100 with each new release.
This strategy, coupled with installment plans from Apple and carriers, has contributed to the iPhone's installed base reaching a record 1.2 billion in 2023, the firm said.
More Tech Stocks:
- Analyst unveils new Facebook stock price target after earnings
- Billionaire George Soros sold this popular semiconductor stock
- Ark’s Cathie Wood just traded 3 popular tech stocks
Apple has effectively shifted its sales mix toward higher-value units despite experiencing slower unit sales, BofA said.
This trend is expected to persist and could help mitigate potential unit sales weaknesses, particularly in China.
BofA also noted Apple's dominance in the high-end market, maintaining a market share of over 90% in the $1,000 and above price band for the past three years.
The firm also cited the anticipation of a multi-year iPhone cycle propelled by next-generation AI technology, robust services growth, and the potential for margin expansion.
On Monday, Evercore ISI analysts said they believed that the sell-off in the iPhone maker’s shares may be “overdone.”
The firm said that investors' growing preference for AI-focused stocks like Nvidia (NVDA) has led to a reallocation of funds away from Apple.
In addition, Evercore said concerns over weakening demand in China, where Apple may be losing market share in the smartphone segment, have affected investor sentiment.
And then ongoing regulatory issues continue to have an impact on investor confidence in the world's second-biggest company.
“We think the sell-off is rather overdone, while we suspect there is strong valuation support at current levels to down 10%, there are three distinct drivers that could unlock upside on the stock from here – a) Cap allocation, b) AI inferencing, and c) Risk-off/defensive shift," the firm said in a research note.
Related: Veteran fund manager picks favorite stocks for 2024
stocks pandemic recovery european europe eu china-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International5 days ago
International5 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International5 days ago
International5 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges





















