International
This device attaches magnetically to a face mask to monitor the wearer’s vitals
Perhaps 2022 will be the year consumer health tracking moves beyond the wrist. We’ve seen Oura’s rise over the past few years and a CES that brought with it a couple of ring fitness trackers. Following Google’s addition to vital and sleep tracking…
Perhaps 2022 will be the year consumer health tracking moves beyond the wrist. We’ve seen Oura’s rise over the past few years and a CES that brought with it a couple of ring fitness trackers. Following Google’s addition to vital and sleep tracking on the Nest Home, Sengled is adding the feature to a smart lightbulb.
So, why not the face mask? Health-related face coverings have long been a fixture in a number of countries, like China, and are pretty much everywhere in this pandemic world. It’s hard to say whether mainstream adoption of masks will outlive COVID-19 in the U.S., but as the pandemic drags on, it seems increasingly likely that they’ll remain a part of daily lives for the foreseeable future.
Image Credits: Northwestern University
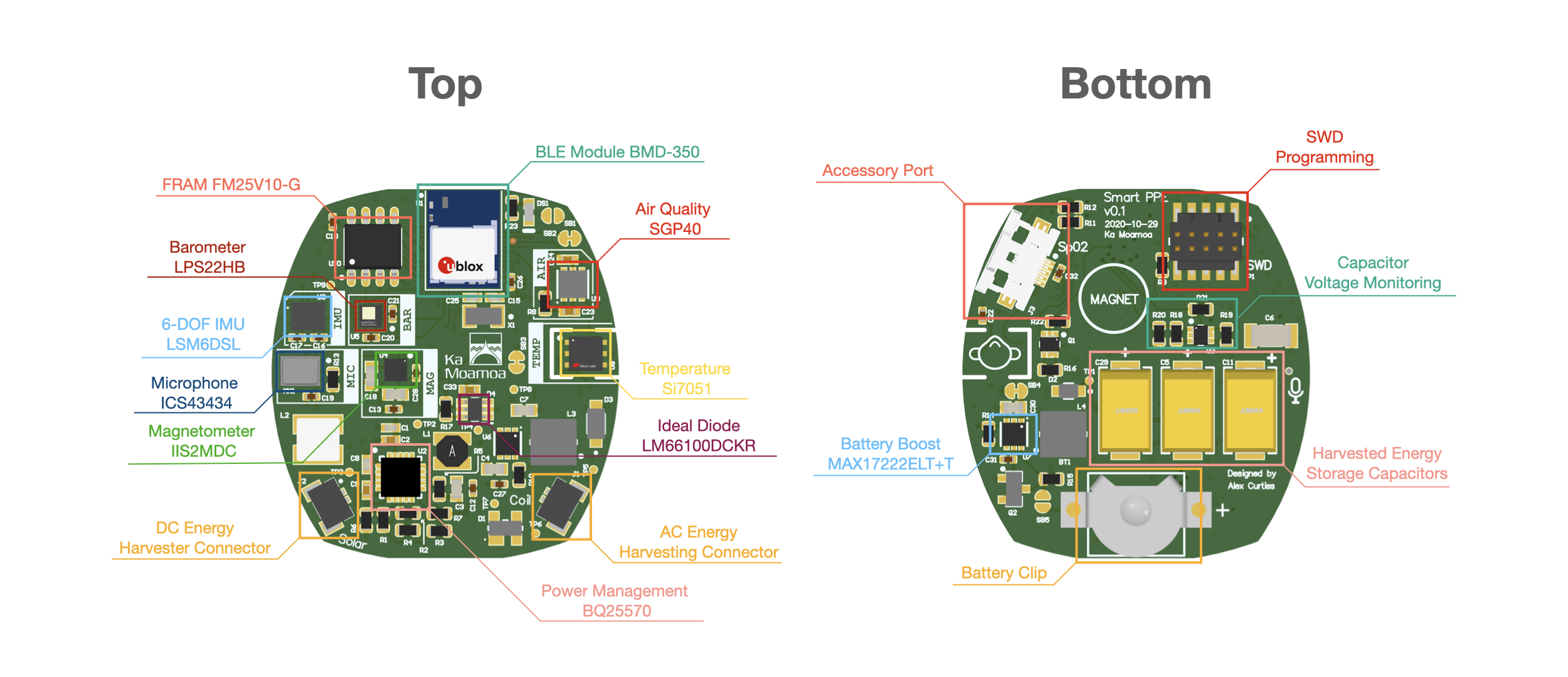
The face is a solid position from which to monitor certain vitals, and the widespread adoption of masks offers a relatively fixed spot to collect that data. Accordingly, a team at Northwestern University is showing off FaceBit — the “FitBit for the Face” — which attaches to an N95, surgical or cloth mask via magnet. From there, it’s able to monitor respiratory and heart rate, as well as time spent in the mask.
“We wanted to design an intelligent face mask for health care professionals that does not need to be inconveniently plugged in during the middle of a shift,” team leader Josiah Hester said in a statement. “We augmented the battery’s energy with energy harvesting from various sources, which means that you can wear the mask for a week or two without having to charge or replace the battery.”
The system, which was recently detailed in a paper, can also determine mask fit — an issue for anyone not accustomed to using a mask. If the mask loosens or is bumped out of place, the connected app will send an alert to the wearer. Currently, the system’s battery lasts around 11 days on a charge, though the team is envisioning a battery-free version, powered by things like thermal and kinetic energy.
The product will need to undergo further clinical trials before proceeding, though the project has also been offered up as an open source product for those interested.
clinical trials pandemic covid-19 chinaInternational
Angry Shouting Aside, Here’s What Biden Is Running On
Angry Shouting Aside, Here’s What Biden Is Running On
Last night, Joe Biden gave an extremely dark, threatening, angry State of the Union…

Last night, Joe Biden gave an extremely dark, threatening, angry State of the Union address - in which he insisted that the American economy is doing better than ever, blamed inflation on 'corporate greed,' and warned that Donald Trump poses an existential threat to the republic.
But in between the angry rhetoric, he also laid out his 2024 election platform - for which additional details will be released on March 11, when the White House sends its proposed budget to Congress.
To that end, Goldman Sachs' Alec Phillips and Tim Krupa have summarized the key points:
Taxes
While railing against billionaires (nothing new there), Biden repeated the claim that anyone making under $400,000 per year won't see an increase in their taxes. He also proposed a 21% corporate minimum tax, up from 15% on book income outlined in the Inflation Reduction Act (IRA), as well as raising the corporate tax rate from 21% to 28% (which would promptly be passed along to consumers in the form of more inflation). Goldman notes that "Congress is unlikely to consider any of these proposals this year, they would only come into play in a second Biden term, if Democrats also won House and Senate majorities."
Biden once again tells the complete lie that "nobody earning less than $400,000/year will pay additional penny in federal taxes."
— RNC Research (@RNCResearch) March 8, 2024
FACT: Biden has *already* raised the tax burden on Americans making as little as $20,000 per year. pic.twitter.com/VrZ1m0rzG3
Biden also called on Congress to restore the pandemic-era child tax credit.
Immigration
Instead of simply passing a slew of border security Executive Orders like the Trump ones he shredded on day one, Biden repeated the lie that Congress 'needs to act' before he can (translation: send money to Ukraine or the US border will continue to be a sieve).
As immigration comes into even greater focus heading into the election, we continue to expect the Administration to tighten policy (e.g., immigration has surged 20pp the last 7 months to first place with 28% in Gallup’s “most important problem” survey). As such, we estimate the foreign-born contribution to monthly labor force growth will moderate from 110k/month in 2023 to around 70-90k/month in 2024. -GS
SEE IT: Biden gets boo-ed while talking about his immigration bill. WATCH pic.twitter.com/O5FmkYx3xM
— Simon Ateba (@simonateba) March 8, 2024
Ukraine
Biden, with House Speaker Mike Johnson doing his best impression of a bobble-head, urged Congress to pass additional assistance for Ukraine based entirely on the premise that Russia 'won't stop' there (and would what, trigger article 5 and WW3 no matter what?), despite the fact that Putin explicitly told Tucker Carlson he has no further ambitions, and in fact seeks a settlement.
‼️ Breaking: Putin wants a negotiated settlement to what’s happening in Ukraine.
— Ed (@EdMagari) February 9, 2024
In a surprising turn of events, Tucker Carlson could be the key to peace, potentially playing a crucial role in ending the current conflict????️ pic.twitter.com/IKN8ajlEUX
As Goldman estimates, "While there is still a clear chance that such a deal could come together, for now there is no clear path forward for Ukraine aid in Congress."
China
Biden, forgetting about all the aggressive tariffs, suggested that Trump had been soft on China, and that he will stand up "against China's unfair economic practices" and "for peace and stability across the Taiwan Strait."
SOTU FACT CHECK:
— Wesley Hunt (@WesleyHuntTX) March 8, 2024
Biden claims we’re in a strong position to take on China.
No president in our lifetime has been WEAKER on China than Biden. pic.twitter.com/Y73JsIzmM3
Healthcare
Lastly, Biden proposed to expand drug price negotiations to 50 additional drugs each year (an increase from 20 outlined in the IRA), which Goldman said would likely require bipartisan support "even if Democrats controlled Congress and the White House," as such policies would likely be ineligible for the budget "reconciliation" process which has been used in previous years to pass the IRA and other major fiscal party when Congressional margins are just too thin.
So there you have it. With no actual accomplishments to speak of, Biden can only attack Trump, lie, and make empty promises.
International
United Airlines adds new flights to faraway destinations
The airline said that it has been working hard to "find hidden gem destinations."

Since countries started opening up after the pandemic in 2021 and 2022, airlines have been seeing demand soar not just for major global cities and popular routes but also for farther-away destinations.
Numerous reports, including a recent TripAdvisor survey of trending destinations, showed that there has been a rise in U.S. traveler interest in Asian countries such as Japan, South Korea and Vietnam as well as growing tourism traction in off-the-beaten-path European countries such as Slovenia, Estonia and Montenegro.
Related: 'No more flying for you': Travel agency sounds alarm over risk of 'carbon passports'
As a result, airlines have been looking at their networks to include more faraway destinations as well as smaller cities that are growing increasingly popular with tourists and may not be served by their competitors.
Shutterstock
United brings back more routes, says it is committed to 'finding hidden gems'
This week, United Airlines (UAL) announced that it will be launching a new route from Newark Liberty International Airport (EWR) to Morocco's Marrakesh. While it is only the country's fourth-largest city, Marrakesh is a particularly popular place for tourists to seek out the sights and experiences that many associate with the country — colorful souks, gardens with ornate architecture and mosques from the Moorish period.
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
"We have consistently been ahead of the curve in finding hidden gem destinations for our customers to explore and remain committed to providing the most unique slate of travel options for their adventures abroad," United's SVP of Global Network Planning Patrick Quayle, said in a press statement.
The new route will launch on Oct. 24 and take place three times a week on a Boeing 767-300ER (BA) plane that is equipped with 46 Polaris business class and 22 Premium Plus seats. The plane choice was a way to reach a luxury customer customer looking to start their holiday in Marrakesh in the plane.
Along with the new Morocco route, United is also launching a flight between Houston (IAH) and Colombia's Medellín on Oct. 27 as well as a route between Tokyo and Cebu in the Philippines on July 31 — the latter is known as a "fifth freedom" flight in which the airline flies to the larger hub from the mainland U.S. and then goes on to smaller Asian city popular with tourists after some travelers get off (and others get on) in Tokyo.
United's network expansion includes new 'fifth freedom' flight
In the fall of 2023, United became the first U.S. airline to fly to the Philippines with a new Manila-San Francisco flight. It has expanded its service to Asia from different U.S. cities earlier last year. Cebu has been on its radar amid growing tourist interest in the region known for marine parks, rainforests and Spanish-style architecture.
With the summer coming up, United also announced that it plans to run its current flights to Hong Kong, Seoul, and Portugal's Porto more frequently at different points of the week and reach four weekly flights between Los Angeles and Shanghai by August 29.
"This is your normal, exciting network planning team back in action," Quayle told travel website The Points Guy of the airline's plans for the new routes.
stocks pandemic south korea japan hong kong europeanInternational
Walmart launches clever answer to Target’s new membership program
The retail superstore is adding a new feature to its Walmart+ plan — and customers will be happy.

It's just been a few days since Target (TGT) launched its new Target Circle 360 paid membership plan.
The plan offers free and fast shipping on many products to customers, initially for $49 a year and then $99 after the initial promotional signup period. It promises to be a success, since many Target customers are loyal to the brand and will go out of their way to shop at one instead of at its two larger peers, Walmart and Amazon.
Related: Walmart makes a major price cut that will delight customers
And stop us if this sounds familiar: Target will rely on its more than 2,000 stores to act as fulfillment hubs.
This model is a proven winner; Walmart also uses its more than 4,600 stores as fulfillment and shipping locations to get orders to customers as soon as possible.
Sometimes, this means shipping goods from the nearest warehouse. But if a desired product is in-store and closer to a customer, it reduces miles on the road and delivery time. It's a kind of logistical magic that makes any efficiency lover's (or retail nerd's) heart go pitter patter.
Walmart rolls out answer to Target's new membership tier
Walmart has certainly had more time than Target to develop and work out the kinks in Walmart+. It first launched the paid membership in 2020 during the height of the pandemic, when many shoppers sheltered at home but still required many staples they might ordinarily pick up at a Walmart, like cleaning supplies, personal-care products, pantry goods and, of course, toilet paper.
It also undercut Amazon (AMZN) Prime, which costs customers $139 a year for free and fast shipping (plus several other benefits including access to its streaming service, Amazon Prime Video).
Walmart+ costs $98 a year, which also gets you free and speedy delivery, plus access to a Paramount+ streaming subscription, fuel savings, and more.
If that's not enough to tempt you, however, Walmart+ just added a new benefit to its membership program, ostensibly to compete directly with something Target now has: ultrafast delivery.
Target Circle 360 particularly attracts customers with free same-day delivery for select orders over $35 and as little as one-hour delivery on select items. Target executes this through its Shipt subsidiary.
We've seen this lightning-fast delivery speed only in snippets from Amazon, the king of delivery efficiency. Who better to take on Target, though, than Walmart, which is using a similar store-as-fulfillment-center model?
"Walmart is stepping up to save our customers even more time with our latest delivery offering: Express On-Demand Early Morning Delivery," Walmart said in a statement, just a day after Target Circle 360 launched. "Starting at 6 a.m., earlier than ever before, customers can enjoy the convenience of On-Demand delivery."
Walmart (WMT) clearly sees consumers' desire for near-instant delivery, which obviously saves time and trips to the store. Rather than waiting a day for your order to show up, it might be on your doorstep when you wake up.
Consumers also tend to spend more money when they shop online, and they remain stickier as paying annual members. So, to a growing number of retail giants, almost instant gratification like this seems like something worth striving for.
Related: Veteran fund manager picks favorite stocks for 2024
stocks pandemic mexico-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International11 hours ago
International11 hours agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges



















