NRx Pharmaceuticals Provides Business Update and Reports Second Quarter 2022 Results Focusing on Reactivation & Advancement of its Psychiatry Franchise
NRx Pharmaceuticals Provides Business Update and Reports Second Quarter 2022 Results Focusing on Reactivation & Advancement of its Psychiatry Franchise
PR Newswire
RADNOR, Pa., Aug. 15, 2022
Company to Host Conference Call and Webcast August 15…

NRx Pharmaceuticals Provides Business Update and Reports Second Quarter 2022 Results Focusing on Reactivation & Advancement of its Psychiatry Franchise
PR Newswire
RADNOR, Pa., Aug. 15, 2022
Company to Host Conference Call and Webcast August 15, 2022, at 8:30am ET
RADNOR, Pa., Aug. 15, 2022 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP), ("NRx Pharmaceuticals" or the "Company"), a clinical-stage biopharmaceutical company, today announced its financial results for the second quarter of 2022 and provided a business and clinical update.
"During our second quarter, we reactivated clinical development in our psychiatry franchise around our lead compound, NRX-101. NRX-101 has been awarded Breakthrough Therapy designation and a Special Protocol Agreement for severe bipolar depression with acute suicidal ideation and behavior (ASIB) by the U.S. Food and Drug Administration (FDA), said Stephen Willard, Chief Executive Officer of the Company. "We anticipate initiation of the study in severe bipolar depression with acute suicidal ideation and behavior (ASIB) to commence at the end of 2022 or at the beginning of 2023.
During this quarter, we commenced enrollment in our Phase 2 trial of NRX-101 in patients with Bipolar Depression and Sub-Acute Suicidal Ideation & Behavior (SSIB)", said Willard. "The purpose of this trial is to expand our potential indication from bipolar depression in patients with acute suicidality to the significantly larger population of patients with bipolar depression and sub-acute suicidality, who are being treated in the ambulatory setting. We are evaluating the adequacy of the protocol to support approval for the treatment of the larger SSIB population. We project a readout of the data from this trial by the end of the year or early Q1 of next year".
"We expect to release commercial-stage material of NRX-101 in the coming weeks. This material will be used in our phase 2b/3 trial of NRX-101 for severe bipolar depression in patients with acute suicidal ideation & behavior (ASIB) under the FDA Special Protocol Agreement, which we expect to start in Q4," said Mr. Willard.
Many approved drugs for bipolar depression have warning labels for increased risk of suicide. To our knowledge, NRX-101 is the only oral antidepressant in the bipolar segment that targets patients with active suicidality, which typically is an exclusion criterion in clinical studies of depression and PTSD. Phase 2 STABIL-B trial1 data of NRX-101 demonstrated a significant reduction in both depression and suicidality compared to standard therapy in acutely suicidal patients who were first stabilized with ketamine. We have released non-clinical findings demonstrating that, unlike ketamine, both components in NRX-101 have not shown potential for addiction and are not neurotoxic (i.e., do not cause death of brain cells in FDA-required assays).2
In Q2, we repatriated the manufacture of NRX-101 drug supply to North Carolina and are currently manufacturing clinical supplies for P3 as part of the commercial readiness program.
Our psychiatry franchise builds on a strong scientific and intellectual property foundation with 47 granted patents and 43 pending applications around the world. Our focus is to address this major unmet medical need for which the only currently approved treatment is electroshock therapy. It is estimated that 50% of individuals with bipolar disorder attempt suicide over their lifetime. We believe NRX-101 is a potentially life-saving medicine that could change the treatment paradigm for individuals with bipolar depression that are also experiencing suicidality."
We expect to evaluate the options for ZYESAMI® in COVID-19 Respiratory Failure and other lung disorders once we receive the full data set from NIH towards the end of Q3 or early Q4 and have conducted our own analysis.
Although we are not funding additional clinical trials of ZYESAMI® at this time, we have completed manufacture of phase 3/commercial ready ZYESAMI®. We also received an independent assessment of chest X-ray data from a sub-study that included a subgroup of approximately 80 patients that had survived to day 10 in our Phase 2b/3 study of ZYESAMI®. The sub-study showed a statistically significant improvement in chest X-rays using the RALES score in patients with COVID-19 respiratory failure, compared to a worsening in patients treated with placebo (P<.05). This exploratory data will further guide our assessment of future options for ZYESAMI®.
Key Business & Clinical Highlights
- Announced new leadership with the appointment of Stephen Willard, JD, as CEO and member of the Board of Directors, and Seth Van Voorhees, PhD, MBA, as CFO
- Repositioned company to focus on psychiatry franchise and our Breakthrough Therapy designated drug NRX-101 for Bipolar Depression in Patients with Suicidality. NRX-101 has additionally been awarded a Special Protocol Agreement by the FDA
- Repatriated manufacture of NRX-101 to a leading North Carolina-based manufacturer, completed technology transfer, and manufactured first batch of phase 3/commercial-ready NRX-101 capsules
- Initiated a Phase 2b trial of NRX-101 in patients with Bipolar Depression and Sub-Acute Suicidality (SSIB); 10 planned clinical sites are activated and are actively enrolling patients, with topline data readout anticipated at the end of Q4 22/Q1 23
- Received independent grading of chest x-rays from a subgroup of patients that survived to day 10 from the intravenous ZYESAMI® trial. Top line analysis shows a statistically significant change between baseline and day 10 on the RALES score (i.e., improvement in ZYESAMI®-treated patients and worsening in placebo-treated patients). Ongoing data analysis is continuing.
Financial Results for Quarter ended June 30, 2022
- Research and development expenses for the three months ended June 30, 2022, totaled $3.0 million, compared to $4.7 million for the quarter ended June 30, 2021. The decrease of $1.7 million related primarily to a decrease clinical trials and development expenses related to ZYESAMI®.
- General and administrative expenses for the three months ended June 30, 2022, totaled $6.6 million, compared to $12.5 million for the three months ended June 30, 2021. The decrease of $5.8 million was primarily related to a decrease in stock-based compensation and consultant fees, partially offset by an increase in higher insurance related expenses.
- Other income for the three months ended June 30, 2022, totaled $2.6 million, compared to $17.0 million for the three months ended June 30, 2021. The decrease of $14.4 million primarily related to a decrease in the fair value of certain Substitute Warrants and the Placement Warrants assumed pursuant to the Merger Agreement because of lower stock price levels.
- Net loss for the three months ended June 30, 2022, was $7.0 million compared with a net loss of $0.1 million for the three months ended June 30, 2021.
Financial Results for Six Months ended June 30, 2022
- Research and development expenses for the six months ended June 30, 2022, totaled $8.4 million, compared to $7.6 million for the six months ended June 30, 2021. The increase of $0.9 million related primarily to an increase in regulatory and process development expenses.
- General and administrative expenses for the six months ended June 30, 2022, totaled $16.9 million, compared to $14.6 million for the six months ended June 30, 2021. The increase of $2.3 million was primarily related to an increase in legal, professional and insurance expenses partially offset by a decrease in consultant fees and stock-based compensation expenses.
- Other Income for the six months ended June 30, 2022, totaled $4.9 million, compared to $17.1 million for the recently restated six months ended June 30, 2021. The decrease of $12.2 million primarily related to a decrease in the fair value of certain Substitute Warrants and the Placement Warrants assumed pursuant to the Merger Agreement because of lower stock price levels.
- Net loss for the six months ended June 30, 2022, was $20.4 million compared with a net loss of $25.6 million for the three months ended June 30, 2021.
- As of June 30, 2022, cash was $24.5 million compared to $27.6 million as of December 31, 2021. We believe that we have sufficient funds and if necessary, the ability to reduce expenditures, to support operations through August 2023. The Company may also seek to reduce certain expenditures if needed to reduce cash consumption in support of operations.
Conference Call and Webcast Details
Investors and the general public are invited to listen to a live audio webcast of the conference call, which may be accessed five minutes before the start of the call by dialing (844) 826-3033 (U.S.), (412) 317-5185 (International) Conference ID: 10170239, or through the webcast link NRx Pharmaceuticals Second Quarter 2022 Earnings Call. A replay will be available from the NRx Pharmaceuticals website for thirty days following the call at www.nrxpharma.com.
About NRX-101
Up to 50% of individuals with bipolar disorder attempt suicide over their lifetime, and estimates indicate that up to 20% may succumb to suicide. The only FDA-approved treatment for patients with bipolar depression and acute suicidal ideation & behavior (ASIB) remains electroconvulsive therapy (ECT). Conventional antidepressants can increase the risk of suicide in certain patients; hence their labels contain a warning to that effect. NRX-101 is a patented, fixed dose combination of D-cycloserine and lurasidone, neither of which has shown addiction potential. Based on the results of a Phase II study, NRX-101 received Breakthrough Therapy designation (BTD) from the FDA for the Treatment of Severe Bipolar Depression in Patients with ASIB after initial stabilization with ketamine or other effective therapy.
NRX-101 is one of the first oral antidepressants currently in late-stage clinical studies targeting the NMDA-receptor in the brain, which represents potentially a key new mechanism to treat depression with and without suicidality, as well as PTSD and other indications. To date, NRX-101 is the only oral NMDA investigational medicine focused on bipolar depression in patients with acute and sub-acute suicidality.
NRx Pharmaceuticals expects to begin its registration trial for NRX-101 under a SPA in 4Q 2022.
About NRx Pharmaceuticals
NRx Pharmaceuticals, Inc. draws upon decades of collective, scientific, and drug-development experience applying innovative science to known molecules to address very high unmet needs and bring improved health to patients. NRx Pharmaceuticals is led by executives who have held leadership roles at Lilly, Pfizer, and Novartis as well as major investment banking institutions.
Cautionary Note Regarding Forward-Looking Statements
This announcement of NRx Pharmaceuticals, Inc. includes "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995, which may include, but are not limited to, statements regarding our financial outlook, product development, business prospects, and market and industry trends and conditions, as well as the Company's strategies, plans, objectives, and goals. These forward-looking statements are based on current beliefs, expectations, estimates, forecasts, and projections of, as well as assumptions made by, and information currently available to, the Company's management.
The Company assumes no obligation to revise any forward-looking statement, whether as a result of new information, future events or otherwise. Accordingly, you should not place reliance on any forward-looking statement, and all forward-looking statements are herein qualified by reference to the cautionary statements set forth above.
CORPORATE CONTACT
Molly Cogan
Sr. Director, Global Communications
mcogan@nrxpharma.com
INVESTOR RELATIONS
Tim McCarthy
Investor Relations
tim@lifesciadvisors.com
###tables to follow###
NRX PHARMACEUTICALS, INC. | ||||||||||||
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||||||
(in thousands, except share and per share data) | ||||||||||||
(Unaudited) | ||||||||||||
Three months ended | Six months ended | |||||||||||
June 30, | June 30, | |||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||
Operating expenses: | ||||||||||||
Research and development | $ | 2,958 | $ | 4,659 | $ | 8,441 | $ | 7,568 | ||||
General and administrative | 6,642 | 12,458 | 16,864 | 14,558 | ||||||||
Settlement expense | — | — | — | 21,366 | ||||||||
Reimbursement of expenses from Relief Therapeutics | — | — | — | (771) | ||||||||
Total operating expenses | 9,600 | 17,117 | 25,305 | 42,721 | ||||||||
Loss from operations | (9,600) | (17,117) | (25,305) | (42,721) | ||||||||
Other (income) expenses: | ||||||||||||
Gain on extinguishment of debt | — | — | — | (121) | ||||||||
Interest income | (23) | — | (23) | — | ||||||||
Interest expense | — | 5 | 3 | 10 | ||||||||
Change in fair value of warrant liabilities | (116) | (17,359) | (273) | (17,359) | ||||||||
Change in fair value of Earnout Cash liability | (2,479) | 355 | (4,582) | 355 | ||||||||
Total other (income) expenses | (2,618) | (16,999) | (4,875) | (17,115) | ||||||||
Net loss | (6,982) | (118) | (20,430) | (25,606) | ||||||||
Deemed dividend | — | (255,822) | — | (255,822) | ||||||||
Net loss attributable to common stockholders | $ | (6,982) | $ | (255,940) | $ | (20,430) | $ | (281,428) | ||||
NRX PHARMACEUTICALS, INC. | ||||||
CONDENSED CONSOLIDATED BALANCE SHEETS | ||||||
(in thousands, except share and per share data) | ||||||
June 30, 2022 | December 31, 2021 | |||||
ASSETS | ||||||
Current assets: | ||||||
Cash and cash equivalents | $ | 24,548 | $ | 27,605 | ||
Prepaid expenses and other current assets | 7,866 | 5,109 | ||||
Total current assets | 32,414 | 32,714 | ||||
Other assets | 19 | 15 | ||||
Total assets | $ | 32,433 | $ | 32,729 | ||
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||
Current liabilities: | ||||||
Accounts payable | $ | 3,078 | $ | 3,687 | ||
Accrued and other current liabilities | 3,326 | 2,375 | ||||
Accrued clinical site costs | 658 | 469 | ||||
Earnout Cash liability | — | 4,582 | ||||
Warrant liabilities | 19 | 292 | ||||
Note payable and accrued interest | — | 518 | ||||
Total liabilities | $ | 7,081 | $ | 11,923 | ||
Preferred stock, $0.001 par value, 50,000,000 shares authorized; 0 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively | — | — | ||||
Common stock, $0.001 par value, 500,000,000 shares authorized; 66,641,314 and 58,810,550 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively | 67 | 59 | ||||
Additional paid-in capital | 228,958 | 203,990 | ||||
Accumulated deficit | (203,673) | (183,243) | ||||
Total stockholders' equity | 25,352 | 20,806 | ||||
Total liabilities and stockholders' equity | $ | 32,433 | $ | 32,729 | ||
NRX PHARMACEUTICALS, INC. | ||||||
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||
(in thousands) | ||||||
(Unaudited) | ||||||
Six months ended June 30, | ||||||
2022 | 2021 | |||||
CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||
Net loss | $ | (20,430) | $ | (25,606) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||
Depreciation expense | 2 | 1 | ||||
Stock-based compensation | 2,321 | 4,655 | ||||
Gain on extinguishment of debt | — | (121) | ||||
Change in fair value of warrant liabilities | (273) | (17,359) | ||||
Change in fair value of earnout cash liability | (4,582) | 355 | ||||
Non-cash interest expense | — | 10 | ||||
Non-cash settlement expense | — | 21,366 | ||||
Non-cash consulting expense | — | 4,850 | ||||
Changes in operating assets and liabilities: | ||||||
Account receivable | — | 831 | ||||
Prepaid expenses and other assets | (2,757) | (4,849) | ||||
Accounts payable | (609) | 2,563 | ||||
Accrued expenses and other liabilities | 1,157 | (1,105) | ||||
Net cash used in operating activities | (25,171) | (14,409) | ||||
CASH FLOWS FROM INVESTING ACTIVITIES | ||||||
Purchase of computer equipment | (6) | (3) | ||||
Net cash used in investing activities | (6) | (3) | ||||
CASH FLOWS FROM FINANCING ACTIVITIES | ||||||
Proceeds from issuance of common stock, net of transaction costs | — | 8,489 | ||||
Proceeds from issuance of common stock for exercise of warrant | — | 7,500 | ||||
Effect of Merger, net of transaction costs | — | 11,050 | ||||
Repayment of note payable | (518) | — | ||||
Proceeds from issuance of common stock and warrants issued in private placement, net of issuance costs | 22,638 | — | ||||
Repayment of notes payable assumed in Merger | — | (1,100) | ||||
Repayment of notes payable - related party | — | — | ||||
Net cash provided by financing activities | 22,120 | 25,939 | ||||
Net increase in cash and cash equivalents | (3,057) | 11,527 | ||||
Cash and cash equivalents at beginning of period | 27,605 | 1,859 | ||||
Cash and cash equivalents at end of period | $ | 24,548 | $ | 13,386 | ||
1 NRX-101 (D-cycloserine plus lurasidone) vs. lurasidone for the maintenance of initial stabilization after ketamine in patients with severe bipolar depression with acute suicidal ideation and behavior: A randomized prospective phase 2 trial | medRxiv
2 https://www.biorxiv.org/content/10.1101/2022.06.18.496662v1
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-provides-business-update-and-reports-second-quarter-2022-results-focusing-on-reactivation--advancement-of-its-psychiatry-franchise-301605486.html
SOURCE NRx Pharmaceuticals, Inc.
Government
Survey Shows Declining Concerns Among Americans About COVID-19
Survey Shows Declining Concerns Among Americans About COVID-19
A new survey reveals that only 20% of Americans view covid-19 as "a major threat"…
A new survey reveals that only 20% of Americans view covid-19 as "a major threat" to the health of the US population - a sharp decline from a high of 67% in July 2020.
What's more, the Pew Research Center survey conducted from Feb. 7 to Feb. 11 showed that just 10% of Americans are concerned that they will catch the disease and require hospitalization.
"This data represents a low ebb of public concern about the virus that reached its height in the summer and fall of 2020, when as many as two-thirds of Americans viewed COVID-19 as a major threat to public health," reads the report, which was published March 7.
According to the survey, half of the participants understand the significance of researchers and healthcare providers in understanding and treating long COVID - however 27% of participants consider this issue less important, while 22% of Americans are unaware of long COVID.
What's more, while Democrats were far more worried than Republicans in the past, that gap has narrowed significantly.
"In the pandemic’s first year, Democrats were routinely about 40 points more likely than Republicans to view the coronavirus as a major threat to the health of the U.S. population. This gap has waned as overall levels of concern have fallen," reads the report.
More via the Epoch Times;
The survey found that three in ten Democrats under 50 have received an updated COVID-19 vaccine, compared with 66 percent of Democrats ages 65 and older.
Moreover, 66 percent of Democrats ages 65 and older have received the updated COVID-19 vaccine, while only 24 percent of Republicans ages 65 and older have done so.
“This 42-point partisan gap is much wider now than at other points since the start of the outbreak. For instance, in August 2021, 93 percent of older Democrats and 78 percent of older Republicans said they had received all the shots needed to be fully vaccinated (a 15-point gap),” it noted.
COVID-19 No Longer an Emergency
The U.S. Centers for Disease Control and Prevention (CDC) recently issued its updated recommendations for the virus, which no longer require people to stay home for five days after testing positive for COVID-19.
The updated guidance recommends that people who contracted a respiratory virus stay home, and they can resume normal activities when their symptoms improve overall and their fever subsides for 24 hours without medication.
“We still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses, this includes vaccination, treatment, and staying home when we get sick,” CDC director Dr. Mandy Cohen said in a statement.
The CDC said that while the virus remains a threat, it is now less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease.
“Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19,” it stated.
The federal government suspended its free at-home COVID-19 test program on March 8, according to a website set up by the government, following a decrease in COVID-19-related hospitalizations.
According to the CDC, hospitalization rates for COVID-19 and influenza diseases remain “elevated” but are decreasing in some parts of the United States.
International
Rand Paul Teases Senate GOP Leader Run – Musk Says “I Would Support”
Rand Paul Teases Senate GOP Leader Run – Musk Says "I Would Support"
Republican Kentucky Senator Rand Paul on Friday hinted that he may jump…

Republican Kentucky Senator Rand Paul on Friday hinted that he may jump into the race to become the next Senate GOP leader, and Elon Musk was quick to support the idea. Republicans must find a successor for periodically malfunctioning Mitch McConnell, who recently announced he'll step down in November, though intending to keep his Senate seat until his term ends in January 2027, when he'd be within weeks of turning 86.
So far, the announced field consists of two quintessential establishment types: John Cornyn of Texas and John Thune of South Dakota. While John Barrasso's name had been thrown around as one of "The Three Johns" considered top contenders, the Wyoming senator on Tuesday said he'll instead seek the number two slot as party whip.
Paul used X to tease his potential bid for the position which -- if the GOP takes back the upper chamber in November -- could graduate from Minority Leader to Majority Leader. He started by telling his 5.1 million followers he'd had lots of people asking him about his interest in running...
Thousands of people have been asking if I'd run for Senate leadership...
— Rand Paul (@RandPaul) March 8, 2024
...then followed up with a poll in which he predictably annihilated Cornyn and Thune, taking a 96% share as of Friday night, with the other two below 2% each.
????????️VOTE NOW ????️ ???? Who would you like to be the next Senate leader?
— Rand Paul (@RandPaul) March 8, 2024
Elon Musk was quick to back the idea of Paul as GOP leader, while daring Cornyn and Thune to follow Paul's lead by throwing their names out for consideration by the Twitter-verse X-verse.
I would support Rand Paul and suspect that other candidates will not actually run polls out of concern for the results, but let’s see if they will!
— Elon Musk (@elonmusk) March 8, 2024
Paul has been a stalwart opponent of security-state mass surveillance, foreign interventionism -- to include shoveling billions of dollars into the proxy war in Ukraine -- and out-of-control spending in general. He demonstrated the latter passion on the Senate floor this week as he ridiculed the latest kick-the-can spending package:
This bill is an insult to the American people. The earmarks are all the wasteful spending that you could ever hope to see, and it should be defeated. Read more: https://t.co/Jt8K5iucA4 pic.twitter.com/I5okd4QgDg
— Senator Rand Paul (@SenRandPaul) March 8, 2024
In February, Paul used Senate rules to force his colleagues into a grueling Super Bowl weekend of votes, as he worked to derail a $95 billion foreign aid bill. "I think we should stay here as long as it takes,” said Paul. “If it takes a week or a month, I’ll force them to stay here to discuss why they think the border of Ukraine is more important than the US border.”
Don't expect a Majority Leader Paul to ditch the filibuster -- he's been a hardy user of the legislative delay tactic. In 2013, he spoke for 13 hours to fight the nomination of John Brennan as CIA director. In 2015, he orated for 10-and-a-half-hours to oppose extension of the Patriot Act.
Among the general public, Paul is probably best known as Capitol Hill's chief tormentor of Dr. Anthony Fauci, who was director of the National Institute of Allergy and Infectious Disease during the Covid-19 pandemic. Paul says the evidence indicates the virus emerged from China's Wuhan Institute of Virology. He's accused Fauci and other members of the US government public health apparatus of evading questions about their funding of the Chinese lab's "gain of function" research, which takes natural viruses and morphs them into something more dangerous. Paul has pointedly said that Fauci committed perjury in congressional hearings and that he belongs in jail "without question."
Musk is neither the only nor the first noteworthy figure to back Paul for party leader. Just hours after McConnell announced his upcoming step-down from leadership, independent 2024 presidential candidate Robert F. Kennedy, Jr voiced his support:
Mitch McConnell, who has served in the Senate for almost 40 years, announced he'll step down this November.
— Robert F. Kennedy Jr (@RobertKennedyJr) February 28, 2024
Part of public service is about knowing when to usher in a new generation. It’s time to promote leaders in Washington, DC who won’t kowtow to the military contractors or…
In a testament to the extent to which the establishment recoils at the libertarian-minded Paul, mainstream media outlets -- which have been quick to report on other developments in the majority leader race -- pretended not to notice that Paul had signaled his interest in the job. More than 24 hours after Paul's test-the-waters tweet-fest began, not a single major outlet had brought it to the attention of their audience.
That may be his strongest endorsement yet.
Government
The Great Replacement Loophole: Illegal Immigrants Score 5-Year Work Benefit While “Waiting” For Deporation, Asylum
The Great Replacement Loophole: Illegal Immigrants Score 5-Year Work Benefit While "Waiting" For Deporation, Asylum
Over the past several…
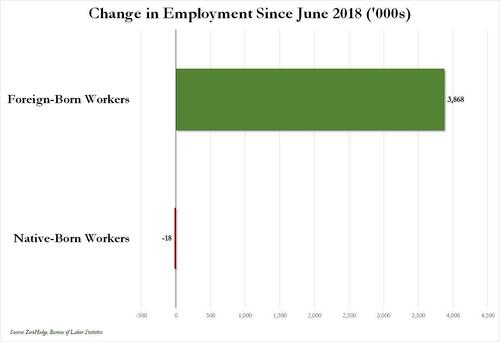
Over the past several months we've pointed out that there has been zero job creation for native-born workers since the summer of 2018...
... and that since Joe Biden was sworn into office, most of the post-pandemic job gains the administration continuously brags about have gone foreign-born (read immigrants, mostly illegal ones) workers.
And while the left might find this data almost as verboten as FBI crime statistics - as it directly supports the so-called "great replacement theory" we're not supposed to discuss - it also coincides with record numbers of illegal crossings into the United States under Biden.
In short, the Biden administration opened the floodgates, 10 million illegal immigrants poured into the country, and most of the post-pandemic "jobs recovery" went to foreign-born workers, of which illegal immigrants represent the largest chunk.

'But Tyler, illegal immigrants can't possibly work in the United States whilst awaiting their asylum hearings,' one might hear from the peanut gallery. On the contrary: ever since Biden reversed a key aspect of Trump's labor policies, all illegal immigrants - even those awaiting deportation proceedings - have been given carte blanche to work while awaiting said proceedings for up to five years...
... something which even Elon Musk was shocked to learn.
Wow, learn something new every day https://t.co/8MDtEEZGam
— Elon Musk (@elonmusk) March 10, 2024
Which leads us to another question: recall that the primary concern for the Biden admin for much of 2022 and 2023 was soaring prices, i.e., relentless inflation in general, and rising wages in particular, which in turn prompted even Goldman to admit two years ago that the diabolical wage-price spiral had been unleashed in the US (diabolical, because nothing absent a major economic shock, read recession or depression, can short-circuit it once it is in place).
Well, there is one other thing that can break the wage-price spiral loop: a flood of ultra-cheap illegal immigrant workers. But don't take our word for it: here is Fed Chair Jerome Powell himself during his February 60 Minutes interview:
PELLEY: Why was immigration important?
POWELL: Because, you know, immigrants come in, and they tend to work at a rate that is at or above that for non-immigrants. Immigrants who come to the country tend to be in the workforce at a slightly higher level than native Americans do. But that's largely because of the age difference. They tend to skew younger.
PELLEY: Why is immigration so important to the economy?
POWELL: Well, first of all, immigration policy is not the Fed's job. The immigration policy of the United States is really important and really much under discussion right now, and that's none of our business. We don't set immigration policy. We don't comment on it.
I will say, over time, though, the U.S. economy has benefited from immigration. And, frankly, just in the last, year a big part of the story of the labor market coming back into better balance is immigration returning to levels that were more typical of the pre-pandemic era.
PELLEY: The country needed the workers.
POWELL: It did. And so, that's what's been happening.
Translation: Immigrants work hard, and Americans are lazy. But much more importantly, since illegal immigrants will work for any pay, and since Biden's Department of Homeland Security, via its Citizenship and Immigration Services Agency, has made it so illegal immigrants can work in the US perfectly legally for up to 5 years (if not more), one can argue that the flood of illegals through the southern border has been the primary reason why inflation - or rather mostly wage inflation, that all too critical component of the wage-price spiral - has moderated in in the past year, when the US labor market suddenly found itself flooded with millions of perfectly eligible workers, who just also happen to be illegal immigrants and thus have zero wage bargaining options.
None of this is to suggest that the relentless flood of immigrants into the US is not also driven by voting and census concerns - something Elon Musk has been pounding the table on in recent weeks, and has gone so far to call it "the biggest corruption of American democracy in the 21st century", but in retrospect, one can also argue that the only modest success the Biden admin has had in the past year - namely bringing inflation down from a torrid 9% annual rate to "only" 3% - has also been due to the millions of illegals he's imported into the country.
We would be remiss if we didn't also note that this so often carries catastrophic short-term consequences for the social fabric of the country (the Laken Riley fiasco being only the latest example), not to mention the far more dire long-term consequences for the future of the US - chief among them the trillions of dollars in debt the US will need to incur to pay for all those new illegal immigrants Democrat voters and low-paid workers. This is on top of the labor revolution that will kick in once AI leads to mass layoffs among high-paying, white-collar jobs, after which all those newly laid off native-born workers hoping to trade down to lower paying (if available) jobs will discover that hardened criminals from Honduras or Guatemala have already taken them, all thanks to Joe Biden.
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 International3 days ago
International3 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex


























