NRx Pharmaceuticals Provides Business Update and Reports Full Year 2021 Financial Results
NRx Pharmaceuticals Provides Business Update and Reports Full Year 2021 Financial Results
PR Newswire
RADNOR, Pa., March 31, 2022
Company to Host Conference Call and Webcast March 31, 2022, at 8:30am ET
RADNOR, Pa., March 31, 2022 /PRNewswire/ — …

NRx Pharmaceuticals Provides Business Update and Reports Full Year 2021 Financial Results
PR Newswire
RADNOR, Pa., March 31, 2022
Company to Host Conference Call and Webcast March 31, 2022, at 8:30am ET
RADNOR, Pa., March 31, 2022 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals"), a clinical-stage, biopharmaceutical company, today announced its financial results for the year ended December 31, 2021 and provided a business update.
"NRx Pharmaceuticals was founded to address high unmet or life-threatening medical needs by applying innovative science to known molecules." said Robert Besthof, Interim Chief Executive Officer. "We are excited to continue to build upon the foundation laid by our founder and my predecessor Dr. Jonathan Javitt. Today NRx is positioned to bring hope to life by executing our strategy in two priority areas in which we have Phase III compounds with Fast Track and Breakthrough Therapy designation, respectively: the development and approval of ZYESAMI® (aviptadil), for treatment of Critical COVID-19 and other respiratory diseases, and advancing our psychiatric portfolio, including NRX-101. Given a changing global geopolitical environment we believe it is prudent to narrow our geographic focus principally in the United States and where we can make the biggest contribution towards improving patient care in the near term. We look forward to advancing the late-stage studies of our promising pipeline of Phase III candidates in Psychiatry and Respiratory Diseases and realizing their potential for patients and investors."
- Announced leadership transition - Robert Besthof named Interim CEO; Dr. Jonathan Javitt to serve as Chief Scientist, member of the Board of Directors
- Company now geographically focused primarily in the U.S. on its two Phase III assets for which it has Fast Track and Breakthrough Therapy designation, respectively: intravenous ZYESAMI® for Critical COVID-19 and NRX-101 for bipolar depression with suicidality
- Re-initiating psychiatry development - initiating a Phase II study of NRX-101 for bipolar depression with sub-acute suicidal ideation and behavior (SSIB); plans to initiate a new Phase IIb/III registrational study of NRX-101 for severe bipolar depression with acute suicidal ideation and behavior (ASIB) using commercial level material in the second half of 2022
- NIH ACTIV-3b Trial of intravenous ZYESAMI® reaching approximately 465 enrolled patients; ZYESAMI is now the only new investigational drug in this study for Critical COVID-19
- I-SPY study of inhaled ZYESAMI® in Critical COVID-19 stopped; NRx inhaled study in severe COVID-19 is paused – exploring inhaled ZYESAMI® for less severe COVID-19 and other respiratory diseases in the future
- Decision made to no longer pursue BriLife™ COVID-19 vaccine project
- Completed $25 million private placement in February 2022
- Research and development expenses for the year ended December 31, 2021, totaled $20.3 million, compared to $10.6 million for the year ended December 31, 2020. The increase was primarily driven by an increase in clinical trials and development expenses related to ZYESAMI®.
- General and administrative expenses for the year ended December 31, 2021, totaled $74.9 million, of which $60.3 million were non-cash stock-based compensation, consulting fees and warrant expense. General and administrative expenses for the year ended December 31, 2020, totaled $11.4 million, of which $5.7 million was non-cash stock-based compensation, consulting fees, and warrant expense. The increase was primarily due to the increase in non-cash stock-based compensation expenses, consulting fees, and an increase in insurance expenses.
- Settlement expense for the year ended December 31, 2021, was $21.4 million compared to $39.5 million for the year ended December 31, 2020. Settlement expense is a non-cash expense.
- Reimbursements of expenses from Relief Therapeutics were $0.8 million for the year ended December 31, 2021, compared to $10.2 million for the year ended December 31, 2020.
- Other income for the year ended December 31, 2021, was $22.7 million, driven primarily by a $20.9 million decrease in the earnout cash liability and a $1.7 million decrease in the warrant liability. Other expenses for the year ended December 31, 2020, were $0.4 million primarily due to a loss on conversion of convertible notes payable.
- Net loss for the year ended December 31, 2021, was $93.1 million, or $1.98 per share, compared with a net loss of $51.8 million, or $1.51 per share for the year ended December 31, 2020.
- Cash used in operating activities was $37.7 million for the year ended December 31, 2021, compared to $2.3 million for the year ended December 31, 2020.
- As of December 31, 2021, cash was $27.6 million, compared to $1.9 million as of December 31, 2020. As previously mentioned, NRx completed a $25 million private placement financing in February 2022. NRx believes it has sufficient cash to support operations for at least the next 12 months.
Investors and the public are invited to listen to a live audio webcast of the conference call, which may be accessed five minutes before the start of the call by dialing (877) 407-9716 (U.S.), (201) 493-6779 (International) Conference ID: 13728080, or through the webcast link NRx Pharmaceuticals Year End Results Call. A replay will be available from the NRx Pharmaceuticals website following the call at www.nrxpharma.com.
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals" or the "Company") draws upon decades of collective, scientific, and drug-development experience to bring improved health to patients. Its investigational product, ZYESAMI® (aviptadil) for patients with COVID-19, has been granted Fast Track designation by the US Food and Drug Administration (FDA) and is in a Phase III trial for Critical COVID-19 patients which is sponsored and managed by the US National Institutes of Health. The FDA has additionally granted Breakthrough Therapy designation, a Special Protocol Agreement, and a Biomarker Letter of Support for NRX-101, an investigational medicine for the treatment of severe bipolar depression in patients with acute suicidal ideation and behavior (ASIB) after initial stabilization with ketamine or other effective therapy.
NRx Pharmaceuticals is led by executives and board members who have held senior roles at Lilly, Pfizer, GSK and the US FDA. NRx Pharmaceuticals was co-founded by Prof Jonathan Javitt, MD, MPH, who has held leadership roles in various biotechnology startup companies and been appointed to advisory roles in four U.S. Presidential Administrations. The NRx Pharmaceuticals' board includes Dr. Sherry Glied, former U.S. Assistant Secretary for Health (ASPE), Daniel E. Troy, JD, former Chief Counsel of the U.S. FDA, Chaim Hurvitz, former director of Teva and President of the Teva International Group, and General H.R. McMaster, Ph.D. (US Army, Ret.), the 26th United States National Security Advisor.
This announcement of NRx Pharmaceuticals, Inc. includes "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995, which may include, but are not limited to, statements regarding our financial outlook, product development, business prospects, and market and industry trends and conditions, as well as the Company's strategies, plans, objectives, and goals. These forward-looking statements are based on current beliefs, expectations, estimates, forecasts, and projections of, as well as assumptions made by, and information currently available to, the Company's management.
The Company assumes no obligation to revise any forward-looking statement, whether as a result of new information, future events or otherwise. Accordingly, you should not place reliance on any forward-looking statement, and all forward-looking statements are herein qualified by reference to the cautionary statements set forth above.
CORPORATE CONTACT | INVESTOR RELATIONS | |
Molly Cogan | Eric Goldstein | |
Senior Director, Global Communications | LifeSci Advisors | |
NRX PHARMACEUTICALS, INC. | ||||||||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||||||||
(in thousands, except share and per share data) | ||||||||||||||
Year ended | ||||||||||||||
December 31, | ||||||||||||||
2021 | 2020 | |||||||||||||
Operating expenses: | ||||||||||||||
Research and development | $ | 20,257 | $ | 10,625 | ||||||||||
General and administrative | 74,944 | 11,436 | ||||||||||||
Settlement expense | 21,366 | 39,486 | ||||||||||||
Reimbursement of expenses from Relief Therapeutics | (771) | (10,160) | ||||||||||||
Total operating expenses | 115,796 | 51,387 | ||||||||||||
Loss from operations | (115,796) | (51,387) | ||||||||||||
Other (income) expenses: | ||||||||||||||
Gain on extinguishment of debt | (121) | — | ||||||||||||
Interest expense | 18 | 56 | ||||||||||||
Change in fair value of warrant liability | (1,692) | — | ||||||||||||
Change in fair value of Earnout Cash liability | (20,938) | — | ||||||||||||
Change in fair value of embedded put | — | 27 | ||||||||||||
Loss on conversion of convertible notes payable | — | 307 | ||||||||||||
Total other (income) expenses | (22,733) | 390 | ||||||||||||
Loss before tax | (93,063) | (51,777) | ||||||||||||
Provision for income taxes | — | — | ||||||||||||
Net loss | (93,063) | (51,777) | ||||||||||||
Deemed dividend - warrants | (2,692) | — | ||||||||||||
Deemed dividend - Earnout Shares | (253,130) | — | ||||||||||||
Net loss attributable to common stockholders | $ | (348,885) | $ | (51,777) | ||||||||||
Net loss per share: | ||||||||||||||
Basic and diluted | $ | (1.98) | $ | (1.51) | ||||||||||
Net loss per share attributable to common stockholders: | ||||||||||||||
Basic and diluted | $ | (7.44) | $ | (1.51) | ||||||||||
Weighted average common shares outstanding: | ||||||||||||||
Basic and diluted | 46,917,701 | 34,270,955 | ||||||||||||
NRX PHARMACEUTICALS, INC. | ||||||||||||
CONSOLIDATED BALANCE SHEETS | ||||||||||||
(in thousands, except share and per share data) | ||||||||||||
December 31, | ||||||||||||
2021 | 2020 | |||||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Cash | $ | 27,605 | $ | 1,859 | ||||||||
Account receivable, net of allowance of $257 as of December 31, 2020 | — | 831 | ||||||||||
Prepaid expenses and other current assets | 5,109 | 240 | ||||||||||
Total current assets | 32,714 | 2,930 | ||||||||||
Other assets | 15 | 11 | ||||||||||
Total assets | $ | 32,729 | $ | 2,941 | ||||||||
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 3,687 | $ | 3,153 | ||||||||
Accrued and other current liabilities | 2,375 | 1,729 | ||||||||||
Accrued clinical site costs | 469 | 1,547 | ||||||||||
Earnout Cash liability | 4,582 | — | ||||||||||
Warrant liabilities | 292 | — | ||||||||||
Notes payable and accrued interest | 518 | 249 | ||||||||||
Accrued settlement expense | — | 39,486 | ||||||||||
Total current liabilities | 11,923 | 46,164 | ||||||||||
Notes payable and accrued interest | — | 548 | ||||||||||
Total liabilities | $ | 11,923 | $ | 46,712 | ||||||||
Stockholders' equity (deficit): | ||||||||||||
Common stock, $0.001 par value, 500,000,000 shares authorized; 58,810,550 | 59 | 43 | ||||||||||
Additional paid-in capital | 203,990 | 46,366 | ||||||||||
Accumulated deficit | (183,243) | (90,180) | ||||||||||
Total stockholders' equity (deficit) | 20,806 | (43,771) | ||||||||||
Total liabilities and stockholders' equity (deficit) | $ | 32,729 | $ | 2,941 | ||||||||
NRX PHARMACEUTICALS, INC. | |||||||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS | |||||||||||||
(in thousands) | |||||||||||||
Year ended December 31, | |||||||||||||
2021 | 2020 | ||||||||||||
CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||||
Net loss | $ | (93,063) | $ | (51,777) | |||||||||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||
Depreciation expense | 2 | 2 | |||||||||||
Stock-based compensation | 7,785 | 730 | |||||||||||
Warrant expense | — | 5,383 | |||||||||||
Gain on extinguishment of debt | (121) | — | |||||||||||
Change in fair value of warrant liabilities | (1,692) | — | |||||||||||
Change in fair value of earnout cash liability | (20,938) | — | |||||||||||
Change in fair value of embedded put | — | 27 | |||||||||||
Amortization of debt discount | — | 17 | |||||||||||
Non-cash interest expense | 19 | 65 | |||||||||||
Non-cash settlement expense | 21,366 | 39,486 | |||||||||||
Non-cash consulting expense | 53,837 | — | |||||||||||
Loss on common stock issued to settle accounts payable | — | 42 | |||||||||||
Loss on conversion for notes payable | — | 307 | |||||||||||
Changes in operating assets and liabilities: | |||||||||||||
Accounts receivable | 831 | (831) | |||||||||||
Prepaid expenses and other assets | (4,809) | (143) | |||||||||||
Accounts payable | (19) | 1,183 | |||||||||||
Accrued expenses and other liabilities | (901) | 3,244 | |||||||||||
Net cash used in operating activities | (37,703) | (2,265) | |||||||||||
CASH FLOWS FROM INVESTING ACTIVITIES | |||||||||||||
Purchase of computer equipment | (7) | (2) | |||||||||||
Net cash used in investing activities | (7) | (2) | |||||||||||
CASH FLOWS FROM FINANCING ACTIVITIES | |||||||||||||
Proceeds from notes payable | — | 620 | |||||||||||
Proceeds from issuance of series B-2 preferred stock | — | 50 | |||||||||||
Proceeds from issuance of common stock and exercise of stock options, net of transaction costs | 9,624 | 2,579 | |||||||||||
Proceeds from issuance of common stock for exercise of warrant | 16,699 | — | |||||||||||
Proceeds from issuance of common stock and warrants issued in private placement, net of issuance costs | 27,359 | — | |||||||||||
Effect of Merger, PIPE financing, net of transaction costs | 11,050 | — | |||||||||||
Repayment of notes payable assumed in Merger | (1,100) | — | |||||||||||
Repayment of notes payable - related party | (176) | — | |||||||||||
Net cash provided by financing activities | 63,456 | 3,249 | |||||||||||
Net increase in cash | 25,746 | 982 | |||||||||||
Cash at beginning of period | 1,859 | 877 | |||||||||||
Cash at end of period | $ | 27,605 | $ | 1,859 | |||||||||
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-provides-business-update-and-reports-full-year-2021-financial-results-301514490.html
SOURCE NRx Pharmaceuticals, Inc.
Uncategorized
After 625 Days, The Longest Yield Curve Inversion In History
After 625 Days, The Longest Yield Curve Inversion In History
Today is a historic day, as last night – DB’s Jim Reid reminds us – we quietly…
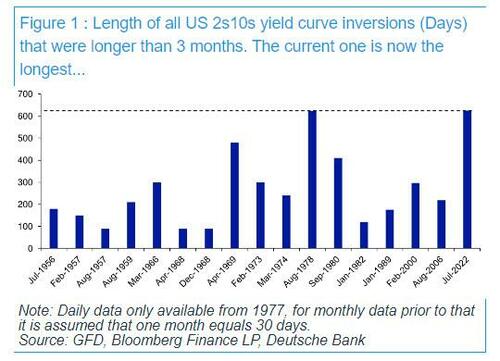
Today is a historic day, as last night - DB's Jim Reid reminds us - we quietly passed the longest continuous US 2s10s inversion in history. After the 2s10s first inverted at the end of March 2022, it has now been continuously inverted for 625 days since July 5th 2022. That exceeds the 624 day inversion from August 1978, which previously held the record.
As regular readers are aware, an inverted yield curve has been the best predictor of a US downturn of any variable through history: the yield curve has always inverted before all of the last 10 US recessions, with a lag that is usually 12-18 months, but some cycles - certainly this one - take longer.... much longer.
In fact, the lack of a recession so far has prompted Red to ask - in his latest Chart of the Day note - if the inverted yield curve recession indicator has failed this cycle?
"Possibly", the DB strategist responds, "but in many ways the yield curve has already accurately predicted many of the drivers that would normally lead to a recession. However, these variables haven't then created recessionary conditions as they normally would have done." He explains:
It led, as it always does, the very sharp deterioration in bank lending standards, and led the declines in bank credit and money supply that are almost unique to this cycle. It was also at the heart of why we had some of the largest bank failures on record with SVB, Signature Bank and First Republic collapsing. A significant part of their failure was a big carry trade that went wrong when the curve inverted.
However, even with the above, a recession - according to the highly political "recession authority" known as the NBER - hasn't materialised. This is perhaps because of the following.
- When lending standards were at their tightest, the borrowing needs of the economy were low relative to previous cycles.
- Excess savings have been unusually high in this cycle (and were revised higher with the GDP revisions last September), so consumers haven't been as exposed to tight credit as they normally are.
- The Fed unveiled a huge series of measures to ensure the regional bank crisis didn't naturally unravel as it would have done in a free market or perhaps in many previous cycles.
- Whilst the Fed’s tightening has been reducing demand, the supply-side of the economy has bounced back strongly from the pandemic disruption, which has further supported growth and made this cycle unique.
So far so good, however, an inverted yield curve should ultimately be a significant headwind for an economy, as capitalism works best when there is a positive return for taking more risk with lending and investments further out the curve. As such, Reid notes, "the rational investor should be prepared to keep more of their money at the front end, or not lend long-term when the curve is inverted" as you are not giving up yield for being able to sleep at night.
So thanks to a historic flood of fiscal stimulus and a daily orgy of new record debt as discussed earlier...
... which means that the US is now running a 6.5% deficit with unemployment near "historical lows", an unheard of event....
... the economy has not succumbed to the inverted yield curve to date, but while it remains inverted the Fed is encouraging more defensive behavior at some point if sentiment changes. As such, the DB strategist concludes that "the quicker we get back to a normal sloping yield curve the safer the system is."
Spread & Containment
Another major retailer cracks down on self-checkout at its stores
The value retailer is discouraging theft at its self-checkout counters by introducing more associate-assisted checkout transactions in its stores.

Huge retail chains like Walmart (WMT) , Target (TGT) , CVS (CVS) and others have faced a high amount of retail theft, or what they call inventory shrink, since 2020 and have been implementing measures to eliminate those costly losses.
Among the most common measures used by Walmart, Target and some others has been locking up popular items behind glass cases to prevent shoplifting. Customers shopping at these stores have encountered a lot of their favorite products, such as cosmetics, shampoo, over-the-counter drugs and even laundry detergent locked up in those cases.
Related: Target limits self-checkout, makes a change customers will love
Shoppers need to either push a button near the product to alert a worker to unlock the case or, in some situations, run around the store looking for a worker with the proper key to open the case. It's a very inconvenient problem for shoppers, and not all stores are consistent with their lockup policies.
For example, one Walmart store might lock up some of their instant coffee products, while another cross-town Walmart location, or even a Target competitor, doesn't lock up any coffee.
Retail stores have also implemented new self-checkout rules to discourage inventory shrink, but again, stores are inconsistent with their rules. Walmart stores have a 20 items or less rule for their self-checkout lanes to try to steer shoppers with more items to checkout clerks that might help reduce the occurrence of theft. But neither customers, nor workers seem to be observing that rule. Target on March 17 implemented a new 10 items or fewer rule in its self-checkout lanes, but we'll see if anyone enforces it.
These self-checkout requirements are also supposed to speed up the checkout process, but that only works if all the self-check registers are working and an adequate amount of checkout clerks are working registers as well.
The next step for retailers in addressing inventory shrink at self-checkout would be to eliminate self-check altogether.
Pat Greenhouse/The Boston Globe via Getty Images
Five Below cuts back on self-checkout lanes
After finishing the fourth quarter of 2023 with a "higher-than-planned shrink," or higher level of theft than expected in its stores, value retailer Five Below (FIVE) has implemented associate-assisted checkout in all of its stores for 2024, CEO Joel Anderson said on the company's earnings call on March 20.
"In addition, in our high-shrink stores, the primary option for checkout is more of the traditional, over-the-counter associate checkout," Anderson said. "We expect to have 75% of our transactions chain-wide assisted by an associate with a goal of 100% in our highest shrink, highest-risk stores to be fully transacted by an associate."
The retailer also checks receipts and adds guards
"Additionally, in those stores, we’re implementing further mitigation efforts, including receipt checking, additional store payroll and guards. We intend to measure progress as soon as Q2 when we perform a limited number of store counts," Anderson said.
Five Below tested several inventory shrink mitigation initiatives late in the third quarter and into the fourth quarter of 2023, which included product-related tests, front-end initiatives and guard programs, Anderson said in the earnings call. He said the most significant change the Philadelphia-based company made across most of the chain was to limit the number of self-checkout registers that were open, while positioning an associate upfront to further assist customers.
Anderson said he is confident the company's measures will help it over time, but the company has not included any financial impact for shrink reduction in its 2024 guidance. The company, however will aggressively pursue returning to pre-pandemic levels of shrink or offsetting the impact over the next few years, he said.
mitigation pandemicGovernment
CCP-Linked Virologist Fired After Transferring Ebola From Winnipeg To Wuhan Resurfaces In China – And Is Collaborating With Military Scientists
CCP-Linked Virologist Fired After Transferring Ebola From Winnipeg To Wuhan Resurfaces In China – And Is Collaborating With Military Scientists
…

A virologist who had a "clandestine relationship" with Chinese agents and was subsequently fired by the Trudeau government has popped back up in China - where she's conducting research with Chinese military scientists and other virology researchers, including at the Wuhan Institute of Virology, where she's allegedly studying antibodies for coronavirus, as well as the deadly Ebola and Niaph viruses, the Globe and Mail reports.
Xiangguo Qiu and her husband Keding Cheng were fired from the National Microbiology Laboratory in Winnipeg, Canada and stripped of their security clearances in July of 2019.
Declassified documents tabled in the House of Commons on Feb. 28 show the couple had provided confidential scientific information to China and posed a credible security threat to the country, according to the Canadian Security Intelligence Service.
The Globe found that Dr. Qiu’s name appears on four Chinese patent filings since 2020, two with the Wuhan Institute of Virology – whose work on bat coronaviruses has placed it at the centre of concerns that it played a role in the spread of COVID-19 – and two with the University of Science and Technology of China, or USTC. The patents relate to antibodies against Nipah virus and work related to nanobodies, including against coronaviruses. -Globe and Mail
Canadian authorities began questioning the pair's loyalty, as well as the potential for coercion or exploitation by a foreign entity, according to more than 600 pages of documents reported by The Counter Signal.
Highlights (via CTVNews.ca):
- Qiu and Cheng were escorted out of Winnipeg's National Microbiology Laboratory in July 2019 and subsequently fired in January 2021.
- The pair transferred deadly Ebola and Henipah viruses to China's Wuhan Institute of Virology in March 2019.
- The Canadian Security Intelligence Service assessed that Qiu repeatedly lied about the extent of her work with institutions of the Chinese government and refused to admit involvement in various Chinese programs, even when evidence was presented to her.
- [D]espite being given every opportunity in her interviews to describe her association with Chinese entities, "Ms. Qiu continued to make blanket denials, feign ignorance or tell outright lies."
- A November 2020 Public Health Agency of Canada report on Qiu says investigators "weighed the adverse information and are in agreement with the CSIS assessment."
- A Public Health Agency report on Cheng's activities says he allowed restricted visitors to work in laboratories unescorted and on at least two occasions did not prevent the unauthorized removal of laboratory materials.
- Cheng was not forthcoming about his activities and collaborations with people from government agencies "of another country, namely members of the People's Republic of China."
Following their firings, Qiu returned to China despite it being under a pandemic travel lockdown until January, 2023.
"It’s very likely that she received quite preferential treatment in China on the basis that she’s proven herself. She’s done a very good job for the government of China," said Brendan Walker-Munro, senior research fellow at Australia’s University of Queensland Law School. "She’s promoted their interests abroad. She’s returned information that is credibly useful to China and to its ongoing research."
More via the Globe and Mail;
Documents reviewed by The Globe show that Dr. Qiu is most closely aligned with the University of Science and Technology of China (USTC) in Hefei. In March, 2023, a document posted by a Chinese pharmaceutical company listed Dr. Qiu as second amongst “major completion personnel” on a project awarded by the Chinese Preventive Medicine Association for study related to an anti-Ebola virus therapeutic antibody. Most of the other completion personnel were associated with the Chinese People’s Liberation Army.
USTC was founded by the Chinese Academy of Sciences and initially established to build up Chinese scientific expertise useful to the military, which at the time was pursuing technology to build satellites, intercontinental ballistic missiles and atomic bombs. The university has continued to maintain close military ties.
The document says Dr. Qiu works for USTC. Jin Tengchuan, the principal investigator at the Laboratory of Structural Immunology at USTC, lists her as a co-inventor on a patent. Mr. Jin did not respond to requests for comment.
A person who answered the phone at USTC told The Globe, “I don’t have any information about this teacher.”
In 2012, USTC signed a strategic co-operation agreement with the Army Engineering University of the People’s Liberation Army, designed to strengthen research on cutting-edge technology useful for communications, weaponry and other national-defence priorities.
Dr. Qiu is also listed as a 2019 doctoral supervisor for students studying virology at Hebei Medical University.
“Well, that makes me wonder what circumstances she was under when she emigrated to Canada. Why did she come?” asked Earl Brown, a professor emeritus of biochemistry, microbiology and immunology at the University of Ottawa’s faculty of medicine who has worked extensively in China in the past. “People leave for more freedom from China, or to make more money. But China keeps tabs on most people so I am not sure if she came over to infiltrate or whether she came and the infiltration happened later through contact with China.”
It may be impossible to answer that question. Three former colleagues at the National Microbiolgy Lab have indicated that Dr. Qiu and her husband were diligent and pleasant to deal with, but largely kept to themselves outside of work. They say Dr. Qiu was a brilliant scientist with a strong work ethic, although her English was weak. The Globe is not identifying the three who did not want to be named.
Dr. Qiu is a medical doctor from Tianjin, China, who came to Canada for graduate studies in 1996. She started at the University of Manitoba, but began working at the national lab as a research scientist in 2006, working her way up to become head of the vaccine development and antiviral therapies section in the National Microbiology Laboratory’s special pathogens program.
She was also part of the team that helped develop ZMapp, a treatment for the deadly Ebola virus, which killed more than 11,000 people in West Africa between 2014 and 2016.
“My sense is this was part of a larger strategy by China to get access to our innovation system,” said Filippa Lentzos, an associate professor of science and international security at King’s College London. “It was a way for them to to find out what was going on in Canada’s premier lab.”
Initially trained as a medical doctor, Dr. Qiu graduated in 1985 from Hebei University in the coastal city of Tianjin, which lies southeast of Beijing. Dr. Qiu went on to obtain her master of science degree in immunology at Tianjin Medical University in 1990.
Her career at Canada’s top infectious disease lab in Winnipeg began in 2003, only four years after Ottawa opened this biosafety level 4 facility at the Canadian Science Centre for Human and Animal Health.
Over time, she built up a reputation for academic collaboration, particularly with China. It was welcomed by management who felt her work was helping build a name internationally for the National Microbiology Lab.
By the time Canadian officials intervened in 2018 and began investigating, documents show, Dr. Qiu was running 44 separate projects at the Winnipeg lab, an uncommonly large workload.
Her work with former colleague and microbiologist Gary Kobinger vaulted Dr. Qiu into the international spotlight. The pair developed a treatment for Ebola, one that in its first human application led to the full recovery of 27 patients with the infection during a 2014 outbreak in Liberia.
Mr. Kobinger’s career continued to soar and he is now director of the Galveston National Laboratory, a renowned biosafety level 4 facility in Texas. In 2022, he told The Globe that it was “heartbreaking” to see what had happened to his colleague. He declined to speak for this article.
“She had lost a lot of weight with all the stress. She was so convinced that this was all a misunderstanding … and she would go back to her job,” he said in 2022. “ Her career has been destroyed with all this. She was one of the top female Canadian scientists of virology and Canada has lost that.”
Over a period of 13 months, though, the Chinese-Canadian microbiologist and her biologist husband’s lives were turned upside down.
She went from being feted at Ottawa’s Rideau Hall with a Governor-General’s Award in May, 2018, to being locked out of the Winnipeg lab in July, 2019 – the high-security facility where she had made her name as a scientist in Canada. By January, 2021, she and Mr. Cheng were fired.
Last month, after being pressed into explaining what happened, the Canadian government finally disclosed the reasons for this extraordinary dismissal: CSIS found the pair had lied about and hid their co-operation with China from Ottawa.
A big question remains following their departure: Why would Dr. Qiu risk her career, including the stature associated with developing an Ebola treatment, for China?
Read the rest here...
-

 Spread & Containment1 week ago
Spread & Containment1 week agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International2 weeks ago
International2 weeks agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized1 month ago
Uncategorized1 month agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 International2 weeks ago
International2 weeks agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized1 month ago
Uncategorized1 month agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized1 month ago
Uncategorized1 month agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges
























