NRx Pharmaceuticals Provides Business Update and Reports First Quarter 2022 Financial Results
NRx Pharmaceuticals Provides Business Update and Reports First Quarter 2022 Financial Results
PR Newswire
RADNOR, Pa., May 16, 2022
Company to Host Conference Call and Webcast May 16, 2022, at 8:30am ET
RADNOR, Pa., May 16, 2022 /PRNewswire/ — NRx…

NRx Pharmaceuticals Provides Business Update and Reports First Quarter 2022 Financial Results
PR Newswire
RADNOR, Pa., May 16, 2022
Company to Host Conference Call and Webcast May 16, 2022, at 8:30am ET
RADNOR, Pa., May 16, 2022 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP, NRx Pharmaceuticals), a clinical-stage biopharmaceutical company, today announced its financial results for the quarter ended March 31, 2022 and provided a business and clinical update.
"During our first quarter of 2022 we continued to make progress on our strategic priorities for our two key compounds, namely advancing NRX-101 for bipolar depression with Acute and Sub-Acute Suicidal Ideation & Behavior and intravenous ZYESAMI® for COVID-19," said Robert Besthof, Interim Chief Executive Officer of NRx Pharmaceuticals.
COVID-19 has also significantly affected the mental health status of the United States. "We have all heard about the recent loss of lives due to suicide of well-known people, and those only known to few. Hence, we are pleased to have restarted development of our psychiatry franchise with the initiation of a Phase II clinical study of NRX-101 for patients with bipolar depression and sub-acute suicidal ideation & behavior (SSIB) for which the first patient was enrolled on May 12, 2022," continued Mr. Besthof. "We expect to initiate our registrational Phase IIb/III study for Bipolar Depression in patients with acute suicidal ideation & behavior (ASIB) in the second half of this year with commercial level material."
Having an effective therapeutic for Critical COVID-19 is of the utmost importance, as the U.S. is still losing nearly 300 Americans to COVID-19 daily. ZYESAMI® is the only remaining drug for Critical COVID-19 in the NIH sponsored ACTIV-3b Trial. NRx Pharmaceuticals made important progress to further improve its proprietary formulation of aviptadil and has now achieved refrigerated stability of up to 8 months and expected multi-year frozen stability (-20°C).
Though infections have gone down since earlier this year, the White House recently issued a warning of up to 100 million new infections in the fall and winter. For the ongoing ZYESAMI® NIH Phase III ACTIV-3b Trial, the next DSMB meeting is scheduled for May 25, 2022, instead of an originally planned meeting in April. The NIH ACTIV-3b Trial leadership indicated to NRx Pharmaceuticals that this new timing would allow the vast majority of patients enrolled to date to have reached the 90-day observation period endpoint. Additionally, the Company submitted filings for Emergency Use Authorization and a Breakthrough Therapy designation request to the U.S. Food & Drug Administration (FDA) both focused on a narrower group of patients with Critical COVID-19, based on a post-hoc analysis of those who progressed despite treatment with remdesivir and other therapies. These filings were supplemented by cumulative safety data of approximately 750 patients treated with intravenous ZYESAMI®. The Company continues to collaborate with the NIH ACTIV-3b Trial leadership in the quest to enable data read out by the end of 2022.
"Overall, we continue to execute on our commitment to bring hope to life by applying innovative science to known molecules in the quest to address very high unmet medical needs," concluded Robert Besthof.
Recent Business Highlights
- Submitted a new Emergency Use Authorization request to the FDA for ZYESAMI® in patients with Critical COVID-19 who are at immediate risk of death from respiratory failure despite treatment with approved therapies including remdesivir.
- Submitted an updated Breakthrough Therapy designation request to the FDA for ZYESAMI® in patients with Critical COVID-19 with respiratory failure that continued to progress despite treatment with remdesivir. This submission included safety data of approximately 750 patients treated with intravenous ZYESAMI® for Critical COVID-19.
- NIH ACTIV-3b Trial DSMB continues to enroll patients and enrollment is expected to rise as infections rise.
- Further advanced commercial manufacturing capabilities for ZYESAMI®, enabling stability of up to eight months, and expected multi-year frozen stability (minus -20°C) based on data to date.
- Restarted development in Psychiatry Franchise by initiating a Phase II study of NRX-101 for bipolar depression with SSIB, and patient enrollment in this study has commenced.
- Preparing to start a new registrational study of NRX-101 for severe bipolar depression with ASIB using commercial level material in the second half of 2022.
- Completed $25 Million private placement in February 2022.
Financial Results for Quarter Ended March 31, 2022
- Research and development expenses for the three months ended March 31, 2022, totaled $5.5 million, compared to $2.9 million for the quarter ended March 31, 2021. The increase of $2.6 million was primarily driven by an increase of $2.1 million in clinical trials and development expenses related to ZYESAMI®.
- General and administrative expenses for the three months ended March 31, 2022, totaled $10.2 million, compared to $2.1 million for the three months ended March 31, 2021. The increase of $8.1 million was primarily related to an increase of $4.4 million in legal, professional, and accounting fees, an increase of $2.2 million in insurance expense, an increase of $0.8 million in stock-based compensation expense and an increase of $0.7 million in other general and administrative expense. The $10.2 million and $2.1 million of general and administrative expenses for the three months ended March 31, 2022, and 2021, respectively, include $1.1 million and $0.3 million, respectively, of non-cash stock-based compensation.
- For the three months ended March 31, 2022, NRx Pharmaceuticals recorded gains of $2.1 million and $0.2 million for the change in fair value of Earnout Cash liability and warrant liability, respectively. NRx Pharmaceuticals recorded no such gains in the three months ended March 31, 2021.
- For the three months ended March 31, 2021, NRx Pharmaceuticals recorded reimbursement of expenses from Relief Therapeutics of $0.8 million, a $0.1 million gain on extinguishment of debt, and a non-cash settlement expense of $21.4 million related to the GEM Warrant. NRX Pharmaceuticals had no reimbursement of expenses from Relief Therapeutics, gain from extinguishment of debt or settlement expense in the three months ended March 31, 2022.
- Net loss for the three months ended March 31, 2022, was $13.4 million, or $0.21 per share, compared with a net loss of $25.5 million, or $0.71 per share for the three months ended March 31, 2021.
- During the three months ended March 31, 2022, NRx Pharmaceuticals used $10.4 million of cash in operating activities compared to $3.0 million during the three months ended March 31, 2021.
- As of March 31, 2022, cash was $40.2 million compared to $27.6 million as of December 31, 2021. NRx Pharmaceuticals believes it has sufficient cash to support operations for at least the next 12 months.
Conference Call and Webcast Details
Investors and the general public are invited to listen to a live audio webcast of the conference call, which may be accessed five minutes before the start of the call by dialing (877) 705-6003 (U.S.), (201) 493-6725 (International) Conference ID: 13729829, or through the webcast link NRx Pharmaceuticals First Quarter 2022 Earnings Call. A replay will be available from the NRx Pharmaceuticals website for thirty days following the call at www.nrxpharma.com.
About NRx Pharmaceuticals
NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals" or the "Company") draws upon decades of collective, scientific, and drug-development experience to bring improved health to patients. The U.S. Food and Drug Administration ("FDA") has granted Breakthrough Therapy designation, a Special Protocol Agreement, and a Biomarker Letter of Support for NRX-101, an investigational medicine for the treatment of severe bipolar depression in patients with acute suicidal ideation and behavior after initial stabilization with ketamine or other effective therapy. In addition, ZYESAMI® (aviptadil), for patients with COVID-19, has been granted Fast Track designation by the FDA and is in a Phase III trial for Critical COVID-19 patients which is sponsored and managed by the US National Institutes of Health.
NRx Pharmaceuticals is led by executives who have held senior leadership roles at Lilly, Pfizer, and Novartis as well as major investment banking institutions.
Cautionary Note Regarding Forward-Looking Statements
This announcement of NRx Pharmaceuticals, Inc. includes "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995, which may include, but are not limited to, statements regarding our financial outlook, product development, business prospects, and market and industry trends and conditions, as well as the Company's strategies, plans, objectives, and goals. These forward-looking statements are based on current beliefs, expectations, estimates, forecasts, and projections of, as well as assumptions made by, and information currently available to, the Company's management.
The Company assumes no obligation to revise any forward-looking statement, whether as a result of new information, future events or otherwise. Accordingly, you should not place reliance on any forward-looking statement, and all forward-looking statements are herein qualified by reference to the cautionary statements set forth above.
CORPORATE CONTACT
Molly Cogan
Head of Global Corporate Communications
mcogan@nrxpharma.com
484.254.6134, ext. 724
INVESTOR RELATIONS
Tom Johnson
Investor Relations
tjohnson@lifesciadvisors.com
--tables to follow---
NRX PHARMACEUTICALS, INC. | |||||||
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | |||||||
(in thousands, except share and per share data) | |||||||
Three months ended | |||||||
March 31, | |||||||
2022 | 2021 | ||||||
Operating expenses: | |||||||
Research and development | $ | 5,483 | $ | 2,909 | |||
General and administrative | 10,222 | 2,101 | |||||
Settlement expense | — | 21,366 | |||||
Reimbursement of expenses from Relief Therapeutics | — | (771) | |||||
Total operating expenses | 15,705 | 25,605 | |||||
Loss from operations | (15,705) | (25,605) | |||||
Other (income) expenses: | |||||||
Gain on extinguishment of debt | — | (121) | |||||
Interest expense | 3 | 5 | |||||
Change in fair value of warrant liability | (157) | — | |||||
Change in fair value of Earnout Cash liability | (2,103) | — | |||||
Total other (income) expenses | (2,257) | (116) | |||||
Net loss | $ | (13,448) | $ | (25,489) | |||
Net loss per share: | |||||||
Basic and diluted | $ | (0.21) | $ | (0.71) | |||
Weighted average common shares outstanding: | |||||||
Basic and diluted | 63,667,468 | 35,658,216 | |||||
NRX PHARMACEUTICALS, INC. | ||||||
CONDENSED CONSOLIDATED BALANCE SHEETS | ||||||
(in thousands, except share and per share data) | ||||||
March 31, 2022 | December 31, 2021 | |||||
(Unaudited) | ||||||
ASSETS | ||||||
Current assets: | ||||||
Cash | $ | 40,202 | $ | 27,605 | ||
Prepaid expenses and other current assets | 3,382 | 5,109 | ||||
Total current assets | 43,584 | 32,714 | ||||
Other assets | 17 | 15 | ||||
Total assets | $ | 43,601 | $ | 32,729 | ||
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||
Current liabilities: | ||||||
Accounts payable | $ | 4,311 | $ | 3,687 | ||
Accrued and other current liabilities | 4,001 | 2,375 | ||||
Accrued clinical site costs | 466 | 469 | ||||
Earnout Cash liability | 2,479 | 4,582 | ||||
Warrant liabilities | 135 | 292 | ||||
Note payable and accrued interest | 520 | 518 | ||||
Total liabilities | $ | 11,912 | $ | 11,923 | ||
Stockholders' equity: | ||||||
Preferred stock, $0.001 par value, 50,000,000 shares authorized; 0 shares | — | — | ||||
Common stock, $0.001 par value, 500,000,000 shares authorized; 66,641,314 | 67 | 59 | ||||
Additional paid-in capital | 228,313 | 203,990 | ||||
Accumulated deficit | (196,691) | (183,243) | ||||
Total stockholders' equity | 31,689 | 20,806 | ||||
Total liabilities and stockholders' equity | $ | 43,601 | $ | 32,729 | ||
NRX PHARMACEUTICALS, INC. | ||||||
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||
(in thousands) | ||||||
(Unaudited) | ||||||
Three months ended March 31, | ||||||
2022 | 2021 | |||||
CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||
Net loss | $ | (13,448) | $ | (25,489) | ||
Adjustments to reconcile net loss to net cash used in operating | ||||||
Depreciation expense | 1 | — | ||||
Stock-based compensation | 1,334 | 372 | ||||
Gain on extinguishment of debt | — | (121) | ||||
Change in fair value of warrant liabilities | (157) | — | ||||
Change in fair value of earnout cash liability | (2,103) | — | ||||
Non-cash interest expense | 2 | 5 | ||||
Non-cash settlement expense | — | 21,366 | ||||
Changes in operating assets and liabilities: | ||||||
Accounts receivable | — | 831 | ||||
Prepaid expenses and other assets | 1,727 | (50) | ||||
Accounts payable | 624 | 1,229 | ||||
Accrued expenses and other liabilities | 1,640 | (1,158) | ||||
Net cash used in operating activities | (10,380) | (3,015) | ||||
CASH FLOWS FROM INVESTING ACTIVITIES | ||||||
Purchase of computer equipment | (3) | — | ||||
Net cash used in investing activities | (3) | — | ||||
CASH FLOWS FROM FINANCING ACTIVITIES | ||||||
Proceeds from issuance of common stock, net of transaction costs | — | 6,927 | ||||
Proceeds from issuance of common stock for exercise of warrant | — | 7,500 | ||||
Proceeds from issuance of common stock and warrants issued in private placement, net of issuance costs | 22,980 | — | ||||
Net cash provided by financing activities | 22,980 | 14,427 | ||||
Net increase in cash | 12,597 | 11,412 | ||||
Cash at beginning of period | 27,605 | 1,859 | ||||
Cash at end of period | $ | 40,202 | $ | 13,271 | ||
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-provides-business-update-and-reports-first-quarter-2022-financial-results-301547641.html
SOURCE NRx Pharmaceuticals, Inc.
Government
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Uncategorized
February Employment Situation
By Paul Gomme and Peter Rupert The establishment data from the BLS showed a 275,000 increase in payroll employment for February, outpacing the 230,000…
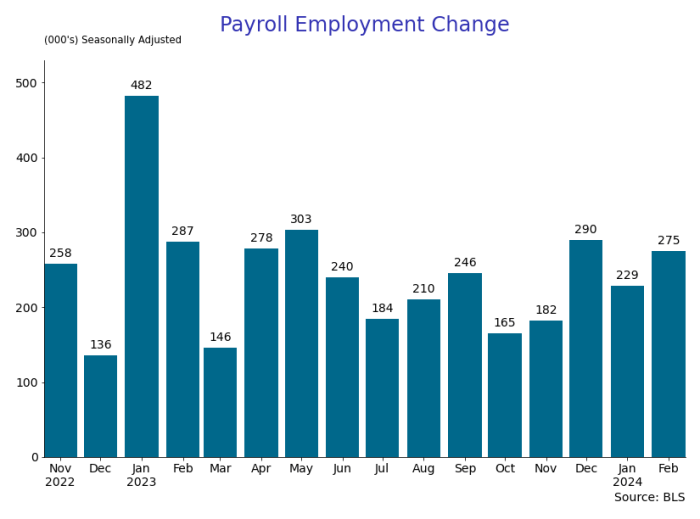
By Paul Gomme and Peter Rupert
The establishment data from the BLS showed a 275,000 increase in payroll employment for February, outpacing the 230,000 average over the previous 12 months. The payroll data for January and December were revised down by a total of 167,000. The private sector added 223,000 new jobs, the largest gain since May of last year.

Temporary help services employment continues a steep decline after a sharp post-pandemic rise.


Average hours of work increased from 34.2 to 34.3. The increase, along with the 223,000 private employment increase led to a hefty increase in total hours of 5.6% at an annualized rate, also the largest increase since May of last year.

The establishment report, once again, beat “expectations;” the WSJ survey of economists was 198,000. Other than the downward revisions, mentioned above, another bit of negative news was a smallish increase in wage growth, from $34.52 to $34.57.

The household survey shows that the labor force increased 150,000, a drop in employment of 184,000 and an increase in the number of unemployed persons of 334,000. The labor force participation rate held steady at 62.5, the employment to population ratio decreased from 60.2 to 60.1 and the unemployment rate increased from 3.66 to 3.86. Remember that the unemployment rate is the number of unemployed relative to the labor force (the number employed plus the number unemployed). Consequently, the unemployment rate can go up if the number of unemployed rises holding fixed the labor force, or if the labor force shrinks holding the number unemployed unchanged. An increase in the unemployment rate is not necessarily a bad thing: it may reflect a strong labor market drawing “marginally attached” individuals from outside the labor force. Indeed, there was a 96,000 decline in those workers.


Earlier in the week, the BLS announced JOLTS (Job Openings and Labor Turnover Survey) data for January. There isn’t much to report here as the job openings changed little at 8.9 million, the number of hires and total separations were little changed at 5.7 million and 5.3 million, respectively.

As has been the case for the last couple of years, the number of job openings remains higher than the number of unemployed persons.

Also earlier in the week the BLS announced that productivity increased 3.2% in the 4th quarter with output rising 3.5% and hours of work rising 0.3%.

The bottom line is that the labor market continues its surprisingly (to some) strong performance, once again proving stronger than many had expected. This strength makes it difficult to justify any interest rate cuts soon, particularly given the recent inflation spike.
unemployment pandemic unemploymentSpread & Containment
Another beloved brewery files Chapter 11 bankruptcy
The beer industry has been devastated by covid, changing tastes, and maybe fallout from the Bud Light scandal.

Before the covid pandemic, craft beer was having a moment. Most cities had multiple breweries and taprooms with some having so many that people put together the brewery version of a pub crawl.
It was a period where beer snobbery ruled the day and it was not uncommon to hear bar patrons discuss the makeup of the beer the beer they were drinking. This boom period always seemed destined for failure, or at least a retraction as many markets seemed to have more craft breweries than they could support.
Related: Fast-food chain closes more stores after Chapter 11 bankruptcy
The pandemic, however, hastened that downfall. Many of these local and regional craft breweries counted on in-person sales to drive their business.
And while many had local and regional distribution, selling through a third party comes with much lower margins. Direct sales drove their business and the pandemic forced many breweries to shut down their taprooms during the period where social distancing rules were in effect.
During those months the breweries still had rent and employees to pay while little money was coming in. That led to a number of popular beermakers including San Francisco's nationally-known Anchor Brewing as well as many regional favorites including Chicago’s Metropolitan Brewing, New Jersey’s Flying Fish, Denver’s Joyride Brewing, Tampa’s Zydeco Brew Werks, and Cleveland’s Terrestrial Brewing filing bankruptcy.
Some of these brands hope to survive, but others, including Anchor Brewing, fell into Chapter 7 liquidation. Now, another domino has fallen as a popular regional brewery has filed for Chapter 11 bankruptcy protection.
Image source: Shutterstock
Covid is not the only reason for brewery bankruptcies
While covid deserves some of the blame for brewery failures, it's not the only reason why so many have filed for bankruptcy protection. Overall beer sales have fallen driven by younger people embracing non-alcoholic cocktails, and the rise in popularity of non-beer alcoholic offerings,
Beer sales have fallen to their lowest levels since 1999 and some industry analysts
"Sales declined by more than 5% in the first nine months of the year, dragged down not only by the backlash and boycotts against Anheuser-Busch-owned Bud Light but the changing habits of younger drinkers," according to data from Beer Marketer’s Insights published by the New York Post.
Bud Light parent Anheuser Busch InBev (BUD) faced massive boycotts after it partnered with transgender social media influencer Dylan Mulvaney. It was a very small partnership but it led to a right-wing backlash spurred on by Kid Rock, who posted a video on social media where he chastised the company before shooting up cases of Bud Light with an automatic weapon.
Another brewery files Chapter 11 bankruptcy
Gizmo Brew Works, which does business under the name Roth Brewing Company LLC, filed for Chapter 11 bankruptcy protection on March 8. In its filing, the company checked the box that indicates that its debts are less than $7.5 million and it chooses to proceed under Subchapter V of Chapter 11.
"Both small business and subchapter V cases are treated differently than a traditional chapter 11 case primarily due to accelerated deadlines and the speed with which the plan is confirmed," USCourts.gov explained.
Roth Brewing/Gizmo Brew Works shared that it has 50-99 creditors and assets $100,000 and $500,000. The filing noted that the company does expect to have funds available for unsecured creditors.
The popular brewery operates three taprooms and sells its beer to go at those locations.
"Join us at Gizmo Brew Works Craft Brewery and Taprooms located in Raleigh, Durham, and Chapel Hill, North Carolina. Find us for entertainment, live music, food trucks, beer specials, and most importantly, great-tasting craft beer by Gizmo Brew Works," the company shared on its website.
The company estimates that it has between $1 and $10 million in liabilities (a broad range as the bankruptcy form does not provide a space to be more specific).
Gizmo Brew Works/Roth Brewing did not share a reorganization or funding plan in its bankruptcy filing. An email request for comment sent through the company's contact page was not immediately returned.
bankruptcy pandemic social distancing
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges



















