International
Moderna booster candidate shows strong response against Omicron subvariants
Moderna Inc. said on Wednesday that an updated version of its COVID-19 vaccine designed to target the Omicron variant also generated a strong immune response…
Moderna booster candidate shows strong response against Omicron subvariants
June 22, 2022, 8:57 AM EDT
June 22 (Reuters) – Moderna Inc. (MRNA.O) said on Wednesday that an updated version of its COVID-19 vaccine designed to target the Omicron variant also generated a strong immune response against the fast-spreading Omicron subvariants BA.4 and BA.5, which have gained a foothold in the U.S. in recent weeks.
The updated vaccine, which Moderna is hoping will be approved for use as a booster shot for the fall, is a bivalent vaccine, meaning it contains vaccine designed to target two different coronavirus variants – the original variant from 2020 and the Omicron variant that was circulating widely last winter.
A Moderna coronavirus disease (COVID-19) vaccine vial is pictured at Skippack Pharmacy in Schwenksville, Pennsylvania, U.S., June 20, 2022. REUTERS/Hannah Beier
Moderna has been producing the updated vaccine on its own dime ahead of any regulatory approvals, and Chief Executive Stéphane Bancel said the company could begin supplying the shot in August.
The company plans to submit applications to regulators in the coming weeks to ask for approval of the shot – which it calls mRNA-1273.214 – for the fall season.
The two sublineages, which were added to the World Health Organization’s monitoring list in March and designated as variants of concern by the European Centre for Disease Prevention and Control, accounted for more than a third of U.S. cases last week.
The U.S. Food and Drug Administration plans to hold a meeting of outside experts next week to discuss the best composition of booster shots for the fall.
Pfizer (PFE.N) and BioNTech (22UAy.DE)are also testing several possible variant-adapted COVID-19 vaccines, including a bivalent candidate similar to Moderna’s.
The European Medicines Agency last week launched a rolling review of their candidates, although the companies have yet to release any data on how well they work. BioNTech this month said market clearance could come as early as August but could also take until September or later in the fall.
Our Standards: The Thomson Reuters Trust Principles.
Source: Reuters
vaccine testing coronavirus covid-19 european world health organizationInternational
Analyst reviews Apple stock price target amid challenges
Here’s what could happen to Apple shares next.

They said it was bound to happen.
It was Jan. 11, 2024 when software giant Microsoft (MSFT) briefly passed Apple (AAPL) as the most valuable company in the world.
Microsoft's stock closed 0.5% higher, giving it a market valuation of $2.859 trillion.
It rose as much as 2% during the session and the company was briefly worth $2.903 trillion. Apple closed 0.3% lower, giving the company a market capitalization of $2.886 trillion.
"It was inevitable that Microsoft would overtake Apple since Microsoft is growing faster and has more to benefit from the generative AI revolution," D.A. Davidson analyst Gil Luria said at the time, according to Reuters.
The two tech titans have jostled for top spot over the years and Microsoft was ahead at last check, with a market cap of $3.085 trillion, compared with Apple's value of $2.684 trillion.
Analysts noted that Apple had been dealing with weakening demand, including for the iPhone, the company’s main source of revenue.
Demand in China, a major market, has slumped as the country's economy makes a slow recovery from the pandemic and competition from Huawei.
Sales in China of Apple's iPhone fell by 24% in the first six weeks of 2024 compared with a year earlier, according to research firm Counterpoint, as the company contended with stiff competition from a resurgent Huawei "while getting squeezed in the middle on aggressive pricing from the likes of OPPO, vivo and Xiaomi," said senior Analyst Mengmeng Zhang.
“Although the iPhone 15 is a great device, it has no significant upgrades from the previous version, so consumers feel fine holding on to the older-generation iPhones for now," he said.
Big plans for China
Counterpoint said that the first six weeks of 2023 saw abnormally high numbers with significant unit sales being deferred from December 2022 due to production issues.
Apple is planning to open its eighth store in Shanghai – and its 47th across China – on March 21.
Related: Tech News Now: OpenAI says Musk contract 'never existed', Xiaomi's EV, and more
The company also plans to expand its research centre in Shanghai to support all of its product lines and open a new lab in southern tech hub Shenzhen later this year, according to the South China Morning Post.
Meanwhile, over in Europe, Apple announced changes to comply with the European Union's Digital Markets Act (DMA), which went into effect last week, Reuters reported on March 12.
Beginning this spring, software developers operating in Europe will be able to distribute apps to EU customers directly from their own websites instead of through the App Store.
"To reflect the DMA’s changes, users in the EU can install apps from alternative app marketplaces in iOS 17.4 and later," Apple said on its website, referring to the software platform that runs iPhones and iPads.
"Users will be able to download an alternative marketplace app from the marketplace developer’s website," the company said.
Apple has also said it will appeal a $2 billion EU antitrust fine for thwarting competition from Spotify (SPOT) and other music streaming rivals via restrictions on the App Store.
The company's shares have suffered amid all this upheaval, but some analysts still see good things in Apple's future.
Bank of America Securities confirmed its positive stance on Apple, maintaining a buy rating with a steady price target of $225, according to Investing.com.
The firm's analysis highlighted Apple's pricing strategy evolution since the introduction of the first iPhone in 2007, with initial prices set at $499 for the 4GB model and $599 for the 8GB model.
BofA said that Apple has consistently launched new iPhone models, including the Pro/Pro Max versions, to target the premium market.
Analyst says Apple selloff 'overdone'
Concurrently, prices for previous models are typically reduced by about $100 with each new release.
This strategy, coupled with installment plans from Apple and carriers, has contributed to the iPhone's installed base reaching a record 1.2 billion in 2023, the firm said.
More Tech Stocks:
- Analyst unveils new Facebook stock price target after earnings
- Billionaire George Soros sold this popular semiconductor stock
- Ark’s Cathie Wood just traded 3 popular tech stocks
Apple has effectively shifted its sales mix toward higher-value units despite experiencing slower unit sales, BofA said.
This trend is expected to persist and could help mitigate potential unit sales weaknesses, particularly in China.
BofA also noted Apple's dominance in the high-end market, maintaining a market share of over 90% in the $1,000 and above price band for the past three years.
The firm also cited the anticipation of a multi-year iPhone cycle propelled by next-generation AI technology, robust services growth, and the potential for margin expansion.
On Monday, Evercore ISI analysts said they believed that the sell-off in the iPhone maker’s shares may be “overdone.”
The firm said that investors' growing preference for AI-focused stocks like Nvidia (NVDA) has led to a reallocation of funds away from Apple.
In addition, Evercore said concerns over weakening demand in China, where Apple may be losing market share in the smartphone segment, have affected investor sentiment.
And then ongoing regulatory issues continue to have an impact on investor confidence in the world's second-biggest company.
“We think the sell-off is rather overdone, while we suspect there is strong valuation support at current levels to down 10%, there are three distinct drivers that could unlock upside on the stock from here – a) Cap allocation, b) AI inferencing, and c) Risk-off/defensive shift," the firm said in a research note.
Related: Veteran fund manager picks favorite stocks for 2024
stocks pandemic recovery european europe eu chinaInternational
Major typhoid fever surveillance study in sub-Saharan Africa indicates need for the introduction of typhoid conjugate vaccines in endemic countries
There is a high burden of typhoid fever in sub-Saharan African countries, according to a new study published today in The Lancet Global Health. This high…
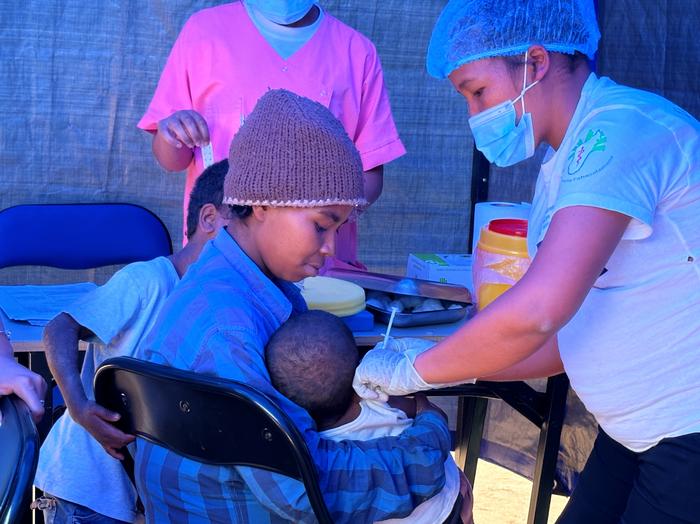
There is a high burden of typhoid fever in sub-Saharan African countries, according to a new study published today in The Lancet Global Health. This high burden combined with the threat of typhoid strains resistant to antibiotic treatment calls for stronger prevention strategies, including the use and implementation of typhoid conjugate vaccines (TCVs) in endemic settings along with improvements in access to safe water, sanitation, and hygiene.
Credit: IVI
There is a high burden of typhoid fever in sub-Saharan African countries, according to a new study published today in The Lancet Global Health. This high burden combined with the threat of typhoid strains resistant to antibiotic treatment calls for stronger prevention strategies, including the use and implementation of typhoid conjugate vaccines (TCVs) in endemic settings along with improvements in access to safe water, sanitation, and hygiene.
The findings from this 4-year study, the Severe Typhoid in Africa (SETA) program, offers new typhoid fever burden estimates from six countries: Burkina Faso, Democratic Republic of the Congo (DRC), Ethiopia, Ghana, Madagascar, and Nigeria, with four countries recording more than 100 cases for every 100,000 person-years of observation, which is considered a high burden. The highest incidence of typhoid was found in DRC with 315 cases per 100,000 people while children between 2-14 years of age were shown to be at highest risk across all 25 study sites.
There are an estimated 12.5 to 16.3 million cases of typhoid every year with 140,000 deaths. However, with generic symptoms such as fever, fatigue, and abdominal pain, and the need for blood culture sampling to make a definitive diagnosis, it is difficult for governments to capture the true burden of typhoid in their countries.
“Our goal through SETA was to address these gaps in typhoid disease burden data,” said lead author Dr. Florian Marks, Deputy Director General of the International Vaccine Institute (IVI). “Our estimates indicate that introduction of TCV in endemic settings would go to lengths in protecting communities, especially school-aged children, against this potentially deadly—but preventable—disease.”
In addition to disease incidence, this study also showed that the emergence of antimicrobial resistance (AMR) in Salmonella Typhi, the bacteria that causes typhoid fever, has led to more reliance beyond the traditional first line of antibiotic treatment. If left untreated, severe cases of the disease can lead to intestinal perforation and even death. This suggests that prevention through vaccination may play a critical role in not only protecting against typhoid fever but reducing the spread of drug-resistant strains of the bacteria.
There are two TCVs prequalified by the World Health Organization (WHO) and available through Gavi, the Vaccine Alliance. In February 2024, IVI and SK bioscience announced that a third TCV, SKYTyphoid™, also achieved WHO PQ, paving the way for public procurement and increasing the global supply.
Alongside the SETA disease burden study, IVI has been working with colleagues in three African countries to show the real-world impact of TCV vaccination. These studies include a cluster-randomized trial in Agogo, Ghana and two effectiveness studies following mass vaccination in Kisantu, DRC and Imerintsiatosika, Madagascar.
Dr. Birkneh Tilahun Tadesse, Associate Director General at IVI and Head of the Real-World Evidence Department, explains, “Through these vaccine effectiveness studies, we aim to show the full public health value of TCV in settings that are directly impacted by a high burden of typhoid fever.” He adds, “Our final objective of course is to eliminate typhoid or to at least reduce the burden to low incidence levels, and that’s what we are attempting in Fiji with an island-wide vaccination campaign.”
As more countries in typhoid endemic countries, namely in sub-Saharan Africa and South Asia, consider TCV in national immunization programs, these data will help inform evidence-based policy decisions around typhoid prevention and control.
###
About the International Vaccine Institute (IVI)
The International Vaccine Institute (IVI) is a non-profit international organization established in 1997 at the initiative of the United Nations Development Programme with a mission to discover, develop, and deliver safe, effective, and affordable vaccines for global health.
IVI’s current portfolio includes vaccines at all stages of pre-clinical and clinical development for infectious diseases that disproportionately affect low- and middle-income countries, such as cholera, typhoid, chikungunya, shigella, salmonella, schistosomiasis, hepatitis E, HPV, COVID-19, and more. IVI developed the world’s first low-cost oral cholera vaccine, pre-qualified by the World Health Organization (WHO) and developed a new-generation typhoid conjugate vaccine that is recently pre-qualified by WHO.
IVI is headquartered in Seoul, Republic of Korea with a Europe Regional Office in Sweden, a Country Office in Austria, and Collaborating Centers in Ghana, Ethiopia, and Madagascar. 39 countries and the WHO are members of IVI, and the governments of the Republic of Korea, Sweden, India, Finland, and Thailand provide state funding. For more information, please visit https://www.ivi.int.
CONTACT
Aerie Em, Global Communications & Advocacy Manager
+82 2 881 1386 | aerie.em@ivi.int
Journal
The Lancet Global Health
Method of Research
Observational study
Subject of Research
People
Article Title
Incidence of typhoid fever in Burkina Faso, Democratic Republic of the Congo, Ethiopia, Ghana, Madagascar, and Nigeria (the Severe Typhoid in Africa programme): a population-based study
Article Publication Date
12-Mar-2024
International
US Spent More Than Double What It Collected In February, As 2024 Deficit Is Second Highest Ever… And Debt Explodes
US Spent More Than Double What It Collected In February, As 2024 Deficit Is Second Highest Ever… And Debt Explodes
Earlier today, CNBC’s…
Earlier today, CNBC's Brian Sullivan took a horse dose of Red Pills when, about six months after our readers, he learned that the US is issuing $1 trillion in debt every 100 days, which prompted him to rage tweet, (or rageX, not sure what the proper term is here) the following:
We’ve added 60% to national debt since 2018. Germany - a country with major economic woes - added ‘just’ 32%.
Maybe it will never matter. Maybe MMT is real. Maybe we just cancel or inflate it out. Maybe career real estate borrowers or career politicians aren’t the answer.
I have no idea. Only time will tell. But it’s going to be fascinating to watch it play out.
He is right: it will be fascinating, and the latest budget deficit data simply confirmed that the day of reckoning will come very soon, certainly sooner than the two years that One River's Eric Peters predicted this weekend for the coming "US debt sustainability crisis."
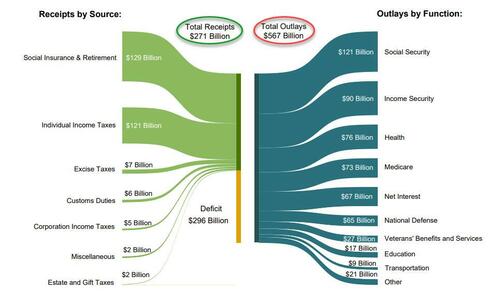
According to the US Treasury, in February, the US collected $271 billion in various tax receipts, and spent $567 billion, more than double what it collected.
The two charts below show the divergence in US tax receipts which have flatlined (on a trailing 6M basis) since the covid pandemic in 2020 (with occasional stimmy-driven surges)...
... and spending which is about 50% higher compared to where it was in 2020.
The end result is that in February, the budget deficit rose to $296.3 billion, up 12.9% from a year prior, and the second highest February deficit on record.
And the punchline: on a cumulative basis, the budget deficit in fiscal 2024 which began on October 1, 2023 is now $828 billion, the second largest cumulative deficit through February on record, surpassed only by the peak covid year of 2021.
But wait there's more: because in a world where the US is spending more than twice what it is collecting, the endgame is clear: debt collapse, and while it won't be tomorrow, or the week after, it is coming... and it's also why the US is now selling $1 trillion in debt every 100 days just to keep operating (and absorbing all those millions of illegal immigrants who will keep voting democrat to preserve the socialist system of the US, so beloved by the Soros clan).
And it gets even worse, because we are now in the ponzi finance stage of the Minsky cycle, with total interest on the debt annualizing well above $1 trillion, and rising every day
... having already surpassed total US defense spending and soon to surpass total health spending and, finally all social security spending, the largest spending category of all, which means that US debt will now rise exponentially higher until the inevitable moment when the US dollar loses its reserve status and it all comes crashing down.
We conclude with another observation by CNBC's Brian Sullivan, who quotes an email by a DC strategist...
.. which lays out the proposed Biden budget as follows:
The budget deficit will growth another $16 TRILLION over next 10 years. Thats *with* the proposed massive tax hikes.
Without them the deficit will grow $19 trillion.
That's why you will hear the "deficit is being reduced by $3 trillion" over the decade.
No family budget or business could exist with this kind of math.
Of course, in the long run, neither can the US... and since neither party will ever cut the spending which everyone by now is so addicted to, the best anyone can do is start planning for the endgame.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International5 days ago
International5 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International5 days ago
International5 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges




























