Government
Deadly fungus Candida auris is spreading across US hospitals – a physician answers 5 questions about rising fungal infections
Candida auris is a relatively new addition to a family of fungi that can infect people. Most of these infections occur in sick, hospitalized patients and…
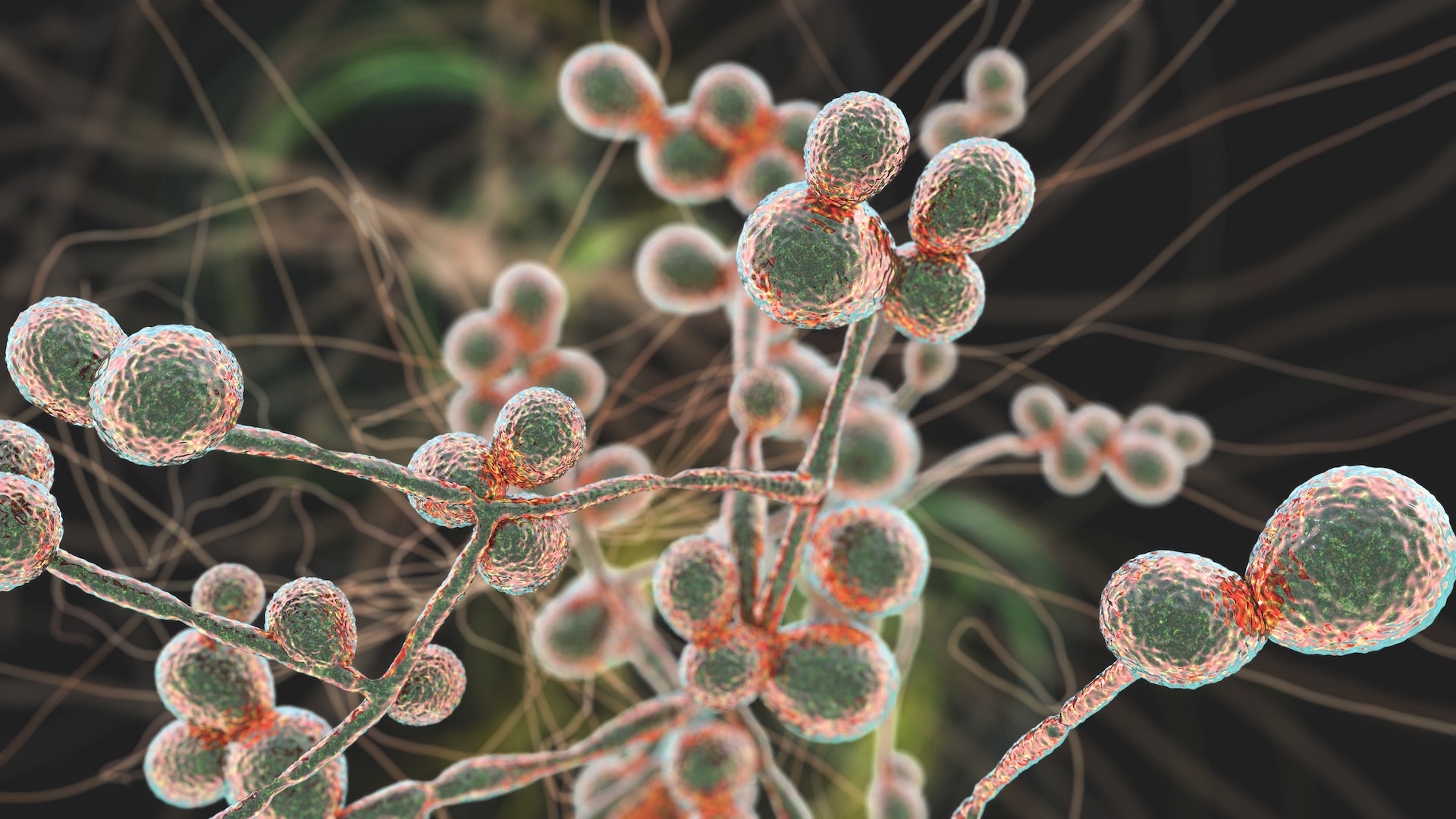
In late March 2023, the U.S. Centers for Disease Control and Prevention highlighted the threat posed by a rapidly spreading fungus called Candida auris that is causing infections and deaths among hospital patients across the country. The unexpected rise of this recently discovered pathogen is part of a larger trend of increasing fungal infections in the U.S.
Arif R. Sarwari is a physician and professor of infectious diseases at West Virginia University. Amid rising concerns among doctors and public health officials, Sarwari helped explain what Candida auris is, how it is spreading and how worried people in the U.S. should be.
1. What is Candida auris?
Candida auris is a recently identified, single-cell fungus that can infect humans and is moderately resistant to existing antifungal drugs. You might be familiar with superficial fungal infections – like athlete’s foot or vaginal yeast infections – which are quite common and don’t pose significant risks to most people. In contrast, Candida auris and other related fungi can cause infections within a person’s body and are much more dangerous.
Candida auris is a type of yeast that was first identified in 2009 and is one of a number of species in the candida family that can infect people. In the past, most invasive candida infections were caused by Candida albicans. Recently, though, infections with species of candida that are much more resistant to drugs than Candida albicans – like Candida auris – have shot up, with a nearly fivefold increase since 2019.

2. How dangerous are candida infections?
For the most part, healthy people do not have to worry about invasive candida infections. There are two groups of people who are most at risk for dangerous candida infections: first are patients in intensive care units who also have central intravenous catheters and are receiving broad spectrum antibiotics. Patients with weak immune systems, such as cancer patients on chemotherapy or patients with human immunodeficiency virus, are also at high risk of candida infection.
Nearly all people have candida fungi growing in their guts and on their skin as part of their microbiome. When a person is healthy, candida numbers are low, but the fungi can proliferate rapidly and overcome a person’s immune system when a patient is sick and on antibiotics.
If candida cells on a person’s skin contaminate an intravenous line, the fungus can get into a patient’s bloodstream and cause often deadly bloodstream infections. Candida species are the fourth most-common cause of hospital associated bloodstream infections.
There are three classes of antifungal drugs that can be used to fight invasive candida infections. Candida albicans is susceptible to all three and easier to treat than Candida auris, which is moderately resistant to all three classes of antifungals.
3. How common are invasive fungal infections?
The CDC estimates that in the U.S., around 25,000 patients get candida bloodstream infections every year.
Candida bloodstream infections are best understood as a tale of two eras. In the past, they were almost always caused by drug-susceptible Candida albicans that arose endogenously from a patient’s own microbiome. There was no concern about infections spreading to other patients.
The recent emergence of drug-resistant and more transmissible Candida auris is raising alarms among health professionals. Because this species can contaminate surfaces and easily spread from patient to patient, the fungus is causing outbreaks both within and between hospitals.
4. Why are fungal infections increasing?
Fungal infections have been rising in the U.S. in recent years, especially infections caused by Candida auris. The pathogen only caused a few infections each year between 2013 and 2016, but starting in 2017, infections began to rise rapidly with 2,377 confirmed cases recorded in 2022 according to the CDC. Deaths caused by all candida infections are rising, too, from 1,010 in 2018 to nearly 1,800 in 2021.
The reasons for this increase are complicated, but I think there are two main drivers: more, sicker patients in hospitals and a stressed health system, both of which got worse during the COVID-19 pandemic.
Hospitals are seeing more very sick patients with weak immune systems, especially as the population ages. This means there are more susceptible patients at hospitals to begin with.
Additionally, any time the health system is stressed – like during a pandemic – drug-resistant bacterial and fungal infections increase. This is because very sick patients are usually in crowded wards and exposed to many antibiotics. In addition, loss of hospital staff and increased workload results in lower quality sanitation - causing more spread of resistant pathogens.
I view the rise of drug-resistant fungi like Candida auris through the same lens as worsening antibiotic resistance. The more antibiotics people use, the greater the chances a resistant strain will become dominant.
5. What can the medical community do about it?
There are a few options for fighting the rise of drug-resistant Candida auris.
The most effective measures are good infection control practices. These behaviors and protocols include practicing good hand hygiene before and after each patient contact, wearing isolation gowns and gloves that are carefully discarded in a patient’s room, and taking measures to detect Candida auris infections early and isolate patients to prevent the spread. Though relatively simple, these actions are key to preventing the spread of all antibiotic-resistant pathogens, not just fungi.
The second option is to develop better drugs to treat new, antifungal-resistant strains of candida. Many new antifungal drugs are already under development. However, prevention through sound infection control will always remain foundational, as further drug development is akin to an arms race.
Arif R. Sarwari does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
cdc disease control pandemic covid-19 spread deaths confirmed casesGovernment
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Government
Walmart joins Costco in sharing key pricing news
The massive retailers have both shared information that some retailers keep very close to the vest.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trumpGovernment
Walmart has really good news for shoppers (and Joe Biden)
The giant retailer joins Costco in making a statement that has political overtones, even if that’s not the intent.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trump-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 International2 days ago
International2 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire





















