Government
Second Wave Shatters COVID-19 Records All Across Europe As France Tops 40k New Cases
COVID-19 Records Shattered Across Europe As France Tops 40k New Cases: Live Updates

Summary:
- France, Italy report new records
- France expands curfew to cover 2/3rds of population
- Netherlands, Denmark suffer records
- Chicago orders 'business curfew'
- Sweden caps night club attendance
- Florida cases top 5,550
- Ohio suffers new record
- Germany, Romania, Poland and Hungary report new records
- US hospitalizations at 2 month high
- Brazil says AZ-Oxford trials to continue
- EU puts pressure on WHO for more transparency
- South Korea sees rise in cases
- Spain warns outbreak "out of control"
* * *
Update (1500ET): More records are being set in Europe Thursday.
France reported more than 40,000 new cases on Thursday (41,622 to be exact), a new record, as French health authorities prepare to expand restrictions beyond Paris and the eight other metro areas where the virus is gaining momentum.
The new curfew measures will cover 2/3rds of the population, doubling the number affected to roughly 46 million.
Ireland, meanwhile, is beginning its 6-week lockdown on Thursday.
New highs were also reported in the Netherlands and Greece on Thursday. In Greece, Prime MInister Kyriakos Mitsotakis announced on Thursday that there would be a curfew in place between 1230 and 0500 in Athens and second-city Thessaloniki.
The Netherlands reported 9,283 new infections, topping 9k for the first time, while in Denmark, officials reported 760 new cases over the last 24 hours, also a record.
Finally, after photos of people partying in Stockholm caused a stir, the Swedish government has enacted a cap on the number of patrons allowed inside nightclubs at 50.
Greece reported a third straight record with 882 new cases bringing the total to 28,216. It also reported 15 more deaths, bringing its death toll to 549.
Florida posted 5,558 new cases on Thursday, its highest daily tally since mid-August.
* * *
Updates (1400ET): As COVID-19 cases continue to climb across the Midwest, Chicago Mayor Lori Lightfoot announced Thursday that the city would be imposing a "business curfew" on bars and restaurants preventing them from serving anybody after 10pm. All nonessential businesses, in fact, will face a 6am to 10pm curfew.
Lightfood said the surge in Chicago's positivity rate over the past week, from just 4.6% to 7%, has given her no other choice. She added that she would "not be shocked" if growing case numbers forced her to implement more emergency measures as time went on.
Elsewhere in the Midwest, Ohio Gov. Mike DeWine reported a single-day record for the state.
Today we are reporting 2,425 new cases since yesterday. This is the highest number we have ever reported on a single day. Of the 10 highest days of new cases reported, eight have occurred in just the past nine days. Nine have occurred in the month of October alone. pic.twitter.com/NYlQHWsjZw
— Governor Mike DeWine (@GovMikeDeWine) October 22, 2020
Gov. DeWine also announced five new "red" counties, bringing the total number of 'red' counties to 38, the largest number yet in the state, as cases and hospitalizations soar.
The new red counties are: Allen, Crawford, Geauga, Hardin, Lake, Lorain, Ottawa, Tuscawaras and Wayne.
— Governor Mike DeWine (@GovMikeDeWine) October 22, 2020
Five of these counties are red for the first time: Crawford, Geauga, Lake, Ottawa and Tuscawaras.
92.8% of Ohioans are living in a county that is red or high incidence. We cannot let our guard down. pic.twitter.com/TW53qSJOvr
— Governor Mike DeWine (@GovMikeDeWine) October 22, 2020
The governor blamed social gatherings and family get-togethers as the primary drivers of the spread, which doesn't bode well ahead of the holiday season.
COVID-19 cases have been surging across the American Midwest, and Europe, which also saw a number of Central European countries, among the hardest hit on the continent, report fresh records on Thursday.
* * *
Thursday is shaping up to be another rough session for Europe in terms of COVID-19, as Germany just reported more than 10,000 new cases (a new record) for one of Europe's best performers, along with Hungary, Romania and Poland, which all reported fresh record numbers of new cases as well.
While cases continued to decline in India, the state of West Bengal notably bucked the trend on Thursday when it reported 4,069 new cases, its biggest daily tally yet, after a major Hindu street festival brought thousands together across the region. All told, India reported just 55,639 new cases in the past day, up from 54,044 the day before. India's death toll jumpd by 702 to 116,616.
Finally, in the US, the number of COVID-19 patients occupying American hospitals hit 40,000 for the first time since August, according to a Reuters tally. The milestone comes as midwestern states like Wisconsin, North Dakota and South Dakota lead the third wave of the US outbreak. Hospitals have seen the number of patients climb 36% over the past 4 weeks. New York reported more than 2,000 cases in a day yesterday for the first time since May.
After a patient enrolled in AstraZeneca's COVID-19 vaccine trial reportedly died, authorities in Brazil said they wouldn't pause the trial, run by AZ and the University of Oxford, after the death of the volunteer. The volunteer was said to be a Brazilian who had received the placebo, suggesting that his death wasn't related to COVID-19 or the trial.
Here's some other big COVID-19 news from overnight and Thursday morning:
German Health Minister Jens Spahn tested positive for the coronavirus, the health ministry says, adding that he had placed himself in home quarantine (Source: Nikkei).
The European Union wants the World Health Organization to become more transparent about how countries report emerging health crises, a draft proposal on reforming the U.N. agency says, according to Reuters. The paper, drawn up by the German government after discussions with other member states following criticism of China's initial handling of the COVID-19 pandemic, is the latest to outline the EU's monthslong plans to address WHO's shortcomings on funding, governance and legal powers (Source: Nikkei).
Tokyo reports 185 new infections, up from 150 the previous day and bringing the capital's total to 29,520.
India's COVID-19 tally tops 7.7 million after 55,839 new cases were reported in the past 24 hours, up from 54,044 the previous day. The death toll jumped by 702 to 116,616.
South Korea confirms 121 new cases, up from 89 a day ago. Total infections reach 25,543 with 453 deaths.
Romania reported a record 4,902 new coronavirus cases in the past 24 hours, bringing the total number of infections to over 196,000. It also registered 98 deaths, the highest daily toll so far. The total number of fatalities stands at 6,163 (Source: Bloomberg).
India’s government has set aside about 500 billion rupees ($7 billion) to vaccinate the world’s most populous nation after China against the coronavirus, according to people with knowledge of the matter (Source: Bloomberg).
Poland registered 12,107 new coronavirus cases in the past 24 hours, a 21% jump from the previous record set a day ago, according to data published by the Health Ministry on Thursday. The death toll in the country of 38 million rose by a record 168 to 4,019. The government is due to announce further restrictions on Thursday in its battle against the pandemic. Slovenia reported a record 1,663 daily infections while the number of hospitalized patients doubled in the past 10 days to 357 (Source: Bloomberg).
One day after becoming the first European country to top 1 million cases, Spain warned that the spread of coronavirus is out of control in certain parts of the country, according to Health Minister Salvador Illa. "We are in the middle of a second wave, it’s no longer a threat but rather a reality," Illa said in an interview on Madrid-based Onda Cero radio. "In some parts of our country the epidemic isn’t under control, so we need to take more drastic measures (Sources: Bloomberg).
Government
Survey Shows Declining Concerns Among Americans About COVID-19
Survey Shows Declining Concerns Among Americans About COVID-19
A new survey reveals that only 20% of Americans view covid-19 as "a major threat"…
A new survey reveals that only 20% of Americans view covid-19 as "a major threat" to the health of the US population - a sharp decline from a high of 67% in July 2020.
What's more, the Pew Research Center survey conducted from Feb. 7 to Feb. 11 showed that just 10% of Americans are concerned that they will catch the disease and require hospitalization.
"This data represents a low ebb of public concern about the virus that reached its height in the summer and fall of 2020, when as many as two-thirds of Americans viewed COVID-19 as a major threat to public health," reads the report, which was published March 7.
According to the survey, half of the participants understand the significance of researchers and healthcare providers in understanding and treating long COVID - however 27% of participants consider this issue less important, while 22% of Americans are unaware of long COVID.
What's more, while Democrats were far more worried than Republicans in the past, that gap has narrowed significantly.
"In the pandemic’s first year, Democrats were routinely about 40 points more likely than Republicans to view the coronavirus as a major threat to the health of the U.S. population. This gap has waned as overall levels of concern have fallen," reads the report.
More via the Epoch Times;
The survey found that three in ten Democrats under 50 have received an updated COVID-19 vaccine, compared with 66 percent of Democrats ages 65 and older.
Moreover, 66 percent of Democrats ages 65 and older have received the updated COVID-19 vaccine, while only 24 percent of Republicans ages 65 and older have done so.
“This 42-point partisan gap is much wider now than at other points since the start of the outbreak. For instance, in August 2021, 93 percent of older Democrats and 78 percent of older Republicans said they had received all the shots needed to be fully vaccinated (a 15-point gap),” it noted.
COVID-19 No Longer an Emergency
The U.S. Centers for Disease Control and Prevention (CDC) recently issued its updated recommendations for the virus, which no longer require people to stay home for five days after testing positive for COVID-19.
The updated guidance recommends that people who contracted a respiratory virus stay home, and they can resume normal activities when their symptoms improve overall and their fever subsides for 24 hours without medication.
“We still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses, this includes vaccination, treatment, and staying home when we get sick,” CDC director Dr. Mandy Cohen said in a statement.
The CDC said that while the virus remains a threat, it is now less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease.
“Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19,” it stated.
The federal government suspended its free at-home COVID-19 test program on March 8, according to a website set up by the government, following a decrease in COVID-19-related hospitalizations.
According to the CDC, hospitalization rates for COVID-19 and influenza diseases remain “elevated” but are decreasing in some parts of the United States.
International
Rand Paul Teases Senate GOP Leader Run – Musk Says “I Would Support”
Rand Paul Teases Senate GOP Leader Run – Musk Says "I Would Support"
Republican Kentucky Senator Rand Paul on Friday hinted that he may jump…

Republican Kentucky Senator Rand Paul on Friday hinted that he may jump into the race to become the next Senate GOP leader, and Elon Musk was quick to support the idea. Republicans must find a successor for periodically malfunctioning Mitch McConnell, who recently announced he'll step down in November, though intending to keep his Senate seat until his term ends in January 2027, when he'd be within weeks of turning 86.
So far, the announced field consists of two quintessential establishment types: John Cornyn of Texas and John Thune of South Dakota. While John Barrasso's name had been thrown around as one of "The Three Johns" considered top contenders, the Wyoming senator on Tuesday said he'll instead seek the number two slot as party whip.
Paul used X to tease his potential bid for the position which -- if the GOP takes back the upper chamber in November -- could graduate from Minority Leader to Majority Leader. He started by telling his 5.1 million followers he'd had lots of people asking him about his interest in running...
Thousands of people have been asking if I'd run for Senate leadership...
— Rand Paul (@RandPaul) March 8, 2024
...then followed up with a poll in which he predictably annihilated Cornyn and Thune, taking a 96% share as of Friday night, with the other two below 2% each.
????????️VOTE NOW ????️ ???? Who would you like to be the next Senate leader?
— Rand Paul (@RandPaul) March 8, 2024
Elon Musk was quick to back the idea of Paul as GOP leader, while daring Cornyn and Thune to follow Paul's lead by throwing their names out for consideration by the Twitter-verse X-verse.
I would support Rand Paul and suspect that other candidates will not actually run polls out of concern for the results, but let’s see if they will!
— Elon Musk (@elonmusk) March 8, 2024
Paul has been a stalwart opponent of security-state mass surveillance, foreign interventionism -- to include shoveling billions of dollars into the proxy war in Ukraine -- and out-of-control spending in general. He demonstrated the latter passion on the Senate floor this week as he ridiculed the latest kick-the-can spending package:
This bill is an insult to the American people. The earmarks are all the wasteful spending that you could ever hope to see, and it should be defeated. Read more: https://t.co/Jt8K5iucA4 pic.twitter.com/I5okd4QgDg
— Senator Rand Paul (@SenRandPaul) March 8, 2024
In February, Paul used Senate rules to force his colleagues into a grueling Super Bowl weekend of votes, as he worked to derail a $95 billion foreign aid bill. "I think we should stay here as long as it takes,” said Paul. “If it takes a week or a month, I’ll force them to stay here to discuss why they think the border of Ukraine is more important than the US border.”
Don't expect a Majority Leader Paul to ditch the filibuster -- he's been a hardy user of the legislative delay tactic. In 2013, he spoke for 13 hours to fight the nomination of John Brennan as CIA director. In 2015, he orated for 10-and-a-half-hours to oppose extension of the Patriot Act.
Among the general public, Paul is probably best known as Capitol Hill's chief tormentor of Dr. Anthony Fauci, who was director of the National Institute of Allergy and Infectious Disease during the Covid-19 pandemic. Paul says the evidence indicates the virus emerged from China's Wuhan Institute of Virology. He's accused Fauci and other members of the US government public health apparatus of evading questions about their funding of the Chinese lab's "gain of function" research, which takes natural viruses and morphs them into something more dangerous. Paul has pointedly said that Fauci committed perjury in congressional hearings and that he belongs in jail "without question."
Musk is neither the only nor the first noteworthy figure to back Paul for party leader. Just hours after McConnell announced his upcoming step-down from leadership, independent 2024 presidential candidate Robert F. Kennedy, Jr voiced his support:
Mitch McConnell, who has served in the Senate for almost 40 years, announced he'll step down this November.
— Robert F. Kennedy Jr (@RobertKennedyJr) February 28, 2024
Part of public service is about knowing when to usher in a new generation. It’s time to promote leaders in Washington, DC who won’t kowtow to the military contractors or…
In a testament to the extent to which the establishment recoils at the libertarian-minded Paul, mainstream media outlets -- which have been quick to report on other developments in the majority leader race -- pretended not to notice that Paul had signaled his interest in the job. More than 24 hours after Paul's test-the-waters tweet-fest began, not a single major outlet had brought it to the attention of their audience.
That may be his strongest endorsement yet.
Government
The Great Replacement Loophole: Illegal Immigrants Score 5-Year Work Benefit While “Waiting” For Deporation, Asylum
The Great Replacement Loophole: Illegal Immigrants Score 5-Year Work Benefit While "Waiting" For Deporation, Asylum
Over the past several…
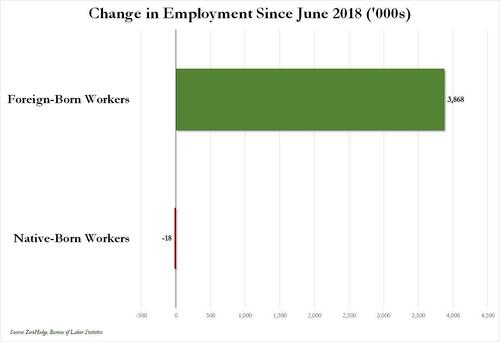
Over the past several months we've pointed out that there has been zero job creation for native-born workers since the summer of 2018...
... and that since Joe Biden was sworn into office, most of the post-pandemic job gains the administration continuously brags about have gone foreign-born (read immigrants, mostly illegal ones) workers.
And while the left might find this data almost as verboten as FBI crime statistics - as it directly supports the so-called "great replacement theory" we're not supposed to discuss - it also coincides with record numbers of illegal crossings into the United States under Biden.
In short, the Biden administration opened the floodgates, 10 million illegal immigrants poured into the country, and most of the post-pandemic "jobs recovery" went to foreign-born workers, of which illegal immigrants represent the largest chunk.

'But Tyler, illegal immigrants can't possibly work in the United States whilst awaiting their asylum hearings,' one might hear from the peanut gallery. On the contrary: ever since Biden reversed a key aspect of Trump's labor policies, all illegal immigrants - even those awaiting deportation proceedings - have been given carte blanche to work while awaiting said proceedings for up to five years...
... something which even Elon Musk was shocked to learn.
Wow, learn something new every day https://t.co/8MDtEEZGam
— Elon Musk (@elonmusk) March 10, 2024
Which leads us to another question: recall that the primary concern for the Biden admin for much of 2022 and 2023 was soaring prices, i.e., relentless inflation in general, and rising wages in particular, which in turn prompted even Goldman to admit two years ago that the diabolical wage-price spiral had been unleashed in the US (diabolical, because nothing absent a major economic shock, read recession or depression, can short-circuit it once it is in place).
Well, there is one other thing that can break the wage-price spiral loop: a flood of ultra-cheap illegal immigrant workers. But don't take our word for it: here is Fed Chair Jerome Powell himself during his February 60 Minutes interview:
PELLEY: Why was immigration important?
POWELL: Because, you know, immigrants come in, and they tend to work at a rate that is at or above that for non-immigrants. Immigrants who come to the country tend to be in the workforce at a slightly higher level than native Americans do. But that's largely because of the age difference. They tend to skew younger.
PELLEY: Why is immigration so important to the economy?
POWELL: Well, first of all, immigration policy is not the Fed's job. The immigration policy of the United States is really important and really much under discussion right now, and that's none of our business. We don't set immigration policy. We don't comment on it.
I will say, over time, though, the U.S. economy has benefited from immigration. And, frankly, just in the last, year a big part of the story of the labor market coming back into better balance is immigration returning to levels that were more typical of the pre-pandemic era.
PELLEY: The country needed the workers.
POWELL: It did. And so, that's what's been happening.
Translation: Immigrants work hard, and Americans are lazy. But much more importantly, since illegal immigrants will work for any pay, and since Biden's Department of Homeland Security, via its Citizenship and Immigration Services Agency, has made it so illegal immigrants can work in the US perfectly legally for up to 5 years (if not more), one can argue that the flood of illegals through the southern border has been the primary reason why inflation - or rather mostly wage inflation, that all too critical component of the wage-price spiral - has moderated in in the past year, when the US labor market suddenly found itself flooded with millions of perfectly eligible workers, who just also happen to be illegal immigrants and thus have zero wage bargaining options.
None of this is to suggest that the relentless flood of immigrants into the US is not also driven by voting and census concerns - something Elon Musk has been pounding the table on in recent weeks, and has gone so far to call it "the biggest corruption of American democracy in the 21st century", but in retrospect, one can also argue that the only modest success the Biden admin has had in the past year - namely bringing inflation down from a torrid 9% annual rate to "only" 3% - has also been due to the millions of illegals he's imported into the country.
We would be remiss if we didn't also note that this so often carries catastrophic short-term consequences for the social fabric of the country (the Laken Riley fiasco being only the latest example), not to mention the far more dire long-term consequences for the future of the US - chief among them the trillions of dollars in debt the US will need to incur to pay for all those new illegal immigrants Democrat voters and low-paid workers. This is on top of the labor revolution that will kick in once AI leads to mass layoffs among high-paying, white-collar jobs, after which all those newly laid off native-born workers hoping to trade down to lower paying (if available) jobs will discover that hardened criminals from Honduras or Guatemala have already taken them, all thanks to Joe Biden.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International3 days ago
International3 days agoWalmart launches clever answer to Target’s new membership program
-

 International3 days ago
International3 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex


























