International
Animal Models and Microfluidic Tools with a Human Touch
Humanized in vivo and in vitro systems are becoming more clinically relevant and facilitating better decisions earlier in drug development.
The post Animal…
Animal models continue to be a mainstay of basic research and preclinical studies. But since animals such as mice only approximate humans, they may generate findings of limited clinical relevance. Fortunately, animal models have become more humanized and much better at generating human-relevant findings, thanks to gene editing technology. Indeed, humanized animal models are readily available from suppliers. Such models include mice that have been engrafted with human cells or engineered to express human gene products.
Alongside the evolution of humanized animal models is the ongoing development of organ-on-a-chip (OOC) models. OOC models range in complexity. Some are single-organ chips. Others are multiorgan microphysiological systems that can capture the subtler mechanisms of systems biology. Accordingly, OOC models are beginning to play a role in drug development and preclinical safety testing, and their future holds even greater promise.
Focusing on immunology
According to Michael Seiler, PhD, vice president, commercial products, Taconic Biosciences, “A heightened focus on immunology as a contributor to systems biology influences how investigators use both immunocompetent and immunodeficient animal models.”
An example of an immunocompetent model is Taconic’s Diet Induced NASH B6, which replicates the progressive and chronic liver inflammation that develops in nonalcoholic steatohepatitis. The NASH B6, which has a fully functioning mouse immune system, can generate predictive results in therapeutic studies. Such models can support studies in various therapeutic areas. Still, an equally strong need exists for immunodeficient animal models, particularly those that gain facets of the human immune system through primary cell engraftments. Such animal models include humanized mice.
“Given the complexity of modeling the human immune system in vivo, we see an increased requirement for optionality in immunodeficient models,” Seiler remarks. “[The most notable options include] the ability to choose the host mouse as well as the primary human cells that will best contribute meaningfully to the experimental design and objective.”
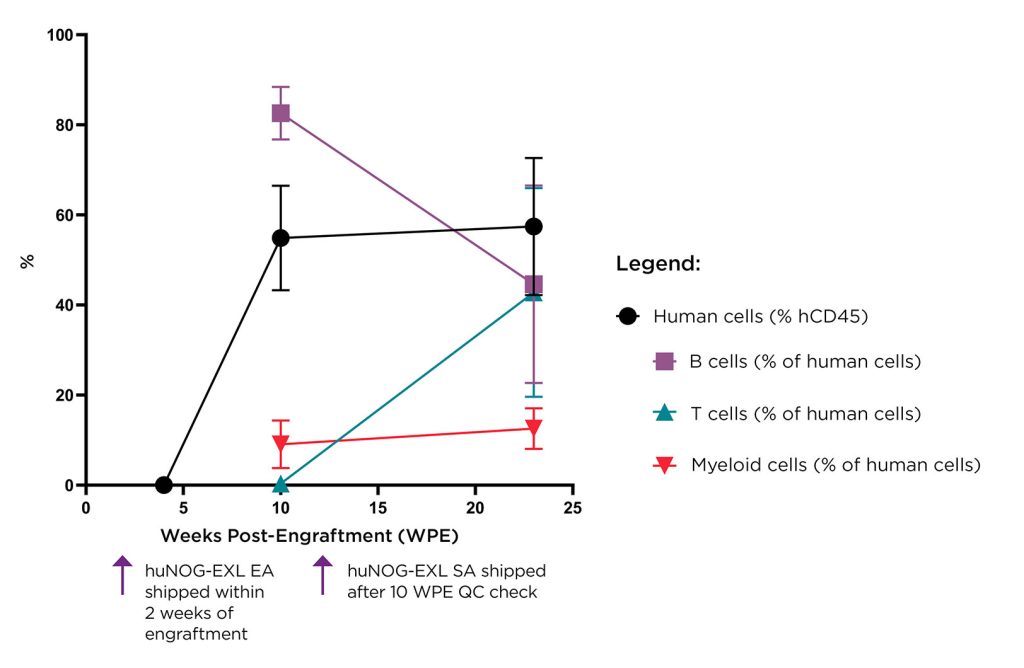
Taconic has expanded its portfolio of humanized models with the addition of the huNOG-EXL EA (early access) mouse model. The huNOG-EXL EA offers a study window that is longer than the one offered by the original huNOG-EXL model, which was launched in 2016. Like many standard humanized models, the huNOG-EXL is typically delivered weeks after engraftment because cell differentiation must be confirmed.
The huNOG-EXL EA retains the advantages of the huNOG-EXL. For example, it supports the differentiation of multiple human cell types known to affect adaptive immunity, including myeloid and lymphoid cells. Also, it is exceptionally reliable and consistent, reflecting Taconic’s experience injecting tens of thousands of mice with hematopoietic stem cells to generate the huNOG-EXL.
In addition, the huNOG-EXL EA can be provided soon after engraftment, prior to the model demonstrating full immune system humanization. This early-access model allows a tumor to be introduced sooner while ensuring that the model’s humanized immune system will mature as expected.
Another recent immunology model, a B-cell-deficient Jh syngeneic model on a B6 background, can help investigators study biologics more efficaciously. For example, the model allows investigators to avoid the complications and risks of anti-drug antibody development.
Taconic sees “exciting opportunities enabled by genetically engineered strains with well-characterized loss of function phenotypes,” Seiler declares. In addition, Taconic will soon announce a microbiome initiative to remove obstacles that have limited investigators to date.
Rethinking animal models
Recent animal model supply chain issues have made it more imperative than ever for researchers to use the most prudent models. Possibilities for moving to more accessible models—that is, models that can be more easily bred, maintained in-house, or even replaced—are now being considered with a greater sense of urgency.
The Jackson Laboratory (JAX) provides researchers worldwide with innovative animal models and preclinical in vivo services. These tools also align with a recently released FDA directive to seek alternatives to nonhuman primates. Several platforms, including immune-humanized mice and genetically diverse models, can address these needs and produce translationally relevant data.
One of the latest platforms from JAX evaluates cytokine release syndrome (CRS). The platform, a peripheral blood mononuclear cell (PBMC)-engrafted humanized mouse, can be used to assess immune-stimulating therapeutics such as chimeric antigen receptor (CAR) T cells and bispecific antibodies. These therapeutics have demonstrated great promise and efficacy, but they can cause PBMCs to overexpress cytokines, giving rise to CRS.
Until recently, the only options for CRS preclinical screening were in vitro platforms and nonhuman primate models. Humanized mouse models, which offer greater speed, accessibility, and versatility, may be more useful.
“We have taken this platform a step further by performing the engraftment in mice lacking MHC class I and II, which dramatically delays the onset of acute graft-versus-host disease,” says Brian Soper, PhD, senior scientific engagement manager, JAX. “This allows performance of longer-term efficacy studies in a model that more closely reflects the human immune system.”
Another JAX initiative is to offer more genetically diverse models such as the J:ARC and HET3 mouse models. “These strains differ drastically,” Soper notes, “but they share value for research and preclinical testing in that their genetic heterogeneity allows us to model likely biological interactions in a diverse population.” Applications range from safety and toxicology screening to quantitative trait locus (QTL) mapping. HET3 also has a unique position in the aging field as it mimics some of the sex differences in aging in the human population.
Genetically diverse platforms facilitate the implementation of precision medicine treatments. For example, these platforms not only inform go/no-go decisions, but they also improve patient stratification, identifying patients for whom treatments might have greater safety and efficacy.
Studying SARS-CoV-2
A challenge of the pandemic has been the lack of animal models that could contribute to the study of human immune cells in SARS-CoV-2 viral infections. Such animal models, however, are starting to become available. For example, Charles River Laboratories has developed humanized mouse models that are susceptible to COVID-19. According to Steve Festin, the company’s senior director of scientific and commercial development, the new models could help investigators understand how different cells in the human immune system respond to early SARS-CoV-2 infection.
In collaboration and under license with GemPharmatech, Charles River recently introduced the hACE2-NCG mouse model. It is based on the triple-immunodeficient NCG mouse, and it reflects the use of a genetic modification strategy that knocks in human ACE2 at the mouse ACE2 locus. (The human ACE2 gene expresses hACE2, the receptor used by SARS-CoV-2 to enter cells.)
“In this model,” Charles River elaborates, “the intracellular domain of the mouse contains ACE2 under the transcriptional regulation of endogenous sequences, designed to mimic the physiological expression pattern of ACE2 in various tissue types, including the kidney, lungs, and intestines.” The company also indicates that its “genetically humanized” model is capable of being “immuno-humanized” with PBMCs or CD34-positive cells.
“As research regains momentum with the majority of laboratories back online, existing trends in oncology discovery and safety are expected to continue, along with greater emphasis on research models supporting cell and gene therapy initiatives,” Festin relates. “We will continue to focus our efforts on providing innovative and meaningful tools for biomedical research and our drug development partners.”
Contextualizing disease correctly
Compounds and targets need to be studied in the correct disease context to ensure a successful development path. Using the wrong models at the start of drug development may very well lead to the wrong drug, as demonstrated by the high attrition of new drug candidates.
Models of healthy and diseased human organs from Mimetas can enable assay development and the screening of thousands of compounds. The company is positioning the models for use in early phases of drug development, including target and compound discovery. Kidney, gut, lung, liver, brain, vasculature, bone marrow, tumor, placenta, and immune system models are available.
The core product is an organ-on-a-chip platform (OrganoPlate). Other products include ready-to-assay 3D tissue models (OrganoReady models), an instrument for taking transepithelial electrical resistance measurements (OrganoTEER), and instruments for driving perfusion flow (OrganoFlow S and OrganoFlow L). Mimetas services include compound profiling and screening services, as well as custom model and assay development services. To further support customers, Mimetas participates in drug development partnerships. Also, the company’s Phenotypic Screening Center is available to facilitate large-scale screening campaigns.

“Recent launches include OrganoStart and OrganoStart Pro packages, OrganoReady blood vessel and vascular bed products, and the high-throughput-screening-compatible OrganoPlate 3-lane 64,” says Paul Vulto, PhD, CEO, Mimetas. “Using 3,500 chips of the OrganoPlate 3-lane 64, we screened 1,546 compounds in duplicate in a 3D angiogenesis assay.1 We also published an assay2 mimicking the onset of inflammation in collaboration with Merck.”
The OrganoPlate comprises proprietary PhaseGuide technology that is designed to allow the horizontal layering of cells and gel matrices without artificial membranes. According to Mimetas, this technology can “enable precise, barrier-free definition of culture matrices and cells in 3D, supporting cell-cell interactions and unprecedented imaging and quantification.” In a recent paper,3 Mimetas scientists asserted that “the co-culture capabilities of the platform can be explored to create complex tissue configurations, for example, by incorporating mesenchymal and immune cells in the ECM adjacent to the epithelial tubes.”
Based on a microtiter plate footprint, the 40- to 96-chip OrganoPlate, comprised of inert materials such as glass and polystyrene, is intended to be fully compatible with microscopes, plate readers, and robotics systems.
“Our flexible suite of offerings, the large array of assays, and the rich ecosystem of instruments around the OrganoPlate make us the go-to company for physiologically relevant modeling,” Vulto asserts. “Throughput and automation of our platform render it well suited for early-stage drug development. Disease context is a crucial piece of the puzzle. Compounds are being progressed or halted based on data from our models.”
Organ-on-a-chip models
“To forward human predictive biology, we build biological models that emulate human physiology more closely,” says Lorna Ewart, PhD, chief scientific officer, Emulate. “Each organ is highly complex. Our philosophy is to start at the simplest level and then layer on complexity.”

The Emulate Organ-Chips employ a polydimethylsiloxane (PDMS) material to create two parallel independent microfluidic channels separated by a porous membrane. Tissue-tissue interfaces are enabled by taking epithelial cells and endothelial cells from the organ of choice, and then adding them to the upper channel and the lower channel, respectively.
Microfluidic technology applies a programmable stream of culture media across the cells simulating sheer stress, an important signal for the cells. “The PDMS also allows us to apply mechanical stress, another important cue for organs such as the lung and intestine,” Ewart notes. “This enhances the physiological relevance.”
Five product lines representing the lung, intestine, liver, kidney, and brain are supplied as Bio-Kits that include the Organ-Chips, Pod Portable Modules, qualified human cells, and chip activation reagents. Bio-Kit resources include validated protocols.
The chips are also offered on a standalone basis for researchers desiring to build their own models. Recent applications for the Colon-Intestine Chip (which incorporates prequalified biopsy-derived primary organoids and colonic endothelial cells) and the Brain-Chip (which incorporates five different cell types) show the introduction and pharmacological modulation of inflammation.
In a recent study, Emulate qualified its Liver-Chip for predictive toxicology. The performance of 780 Liver-Chips was assessed across a blinded set of 27 known hepatotoxic and nontoxic drugs. “We wanted to know how well the human Liver-Chip could predict drug-induced liver injury,” Ewart relates. “Results showed 87% sensitivity and 100% specificity when differentiating hepatotoxic from nonhepatotoxic small molecules.”1
Notably, all the included 22 hepatotoxic drugs had previously been classified as safe due to a lack of toxicity in animal models. Collectively, these compounds resulted in 208 patient fatalities and 10 liver transplants. If the human Liver-Chip had been used during preclinical screening, it is likely that many of these fatalities could have been avoided, Ewart suggests.
A financial framework assessed the economic impact of the technology and showed that the use of the Liver-Chip in pharmaceutical development programs for new candidate drugs could provide the equivalent of $3 billion in improved R&D productivity.4
Multiorgan microphysiological systems
Potential alternatives to animal models include multiorgan microphysiological systems (MPSs). Besides providing a way to implement the 3Rs (Reducing, Replacing, and Refining animal models to satisfy ethical considerations), MPSs may generate in vitro findings that are more clinically relevant than the findings from animal models. The main applications fall within disease modeling; absorption, distribution, metabolism, and excretion (ADME) testing; and toxicology/safety testing.
MPSs from CN Bio Innovations may incorporate several organ-on-a-chip systems, such as systems representing lung, liver, and gut. Systems representing single organs can be linked together into multiorgan systems to simulate processes such as drug absorption and metabolism, or to advance the study of interorgan interactions such as inflammation. CN Bio offers MPSs, validated 3D cell cultures, compatible consumables, and research services. The company maintains that its offerings can address drug development bottlenecks across many therapeutic areas, including metabolic and infectious diseases, oncology, and inflammation.

The PhysioMimix range of single- and multiorgan MPSs harnesses microfluidic technology to mimic blood flow and precisely control the cellular microenvironment. In a collaborative study, CN Bio and FDA scientists demonstrated that data derived using the PhysioMimix liver-on-a-chip system are appropriate for use in drug safety and metabolism applications, evidencing its enhanced performance versus standard in vitro models.5
Many organ-on-a-chip systems currently available complement each other. “There is an inverse relationship between physiological relevance and throughput,” says David Hughes, DPhil, CEO, CN Bio. “Higher-throughput organ-on-a-chip solutions at the start of the screening cascade offer more binary, yes/no decisions. These are perfectly complemented by systems like ours, which can be used for smaller numbers of compounds in preclinical studies where the highest biological and translational relevance is essential.”
“Cultures can be maintained up to four weeks,” Hughes continues, “supporting, for example, studies of how chronic drug dosing may relate to the potential for drug-induced liver injury.” In addition, new, interconnected multiorgan (gut and liver) models recreate human processes in the laboratory such as first-pass metabolism to estimate drug bioavailability.
The open architecture system provides flexibility. New PhysioMimix models are fine-tuned to match their human counterparts as closely as possible through the application of inter- and intraorgan-specific flow rates that deliver human-relevant sheer forces. Purpose-built hardware and consumable plates support tissue-specific requirements such as oxygen gradients essential to the gut microbiome.
References
1. Soragni C, Ng CP, Heijmans J, et al. A robotised 1546 compound screen in a perfused 3D microfluidic angiogenesis assay. Poster presented at SLAS Europe 2021; June 22–25, 2021; Vienna, Austria.
2. De Haan L, Suijker J, van Roey R, et al. A Microfluidic 3D Endothelium-on-a-Chip Model to Study Transendothelial Migration of T Cells in Health and Disease. Int. J. Mol. Sci. 2021; 22(15):8234. DOI: 10.3390/ijms22158234.
3. Trietsch SJ, Naumovska E, Kurek D, et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat. Commun. 2017; 8(1): 262. DOI: 10.1038/s41467-017-00259-3.
4. Ewart L, Apostolou A, Briggs SA, et al. Qualifying a human Liver-Chip for predictive toxicology: Performance assessment and economic implications. bioRxiv. Preprint posted on December 29, 2021. DOI: 10.1101/2021.12.14.472674.
5. Rubiano A, Indapurkar A, Yokosawa R, et al. Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism, and accumulation. Clin. Transl. Sci. 2021; 14: 1049–1061. DOE: 10.1111/cts/12969.
The post Animal Models and Microfluidic Tools with a Human Touch appeared first on GEN - Genetic Engineering and Biotechnology News.
testing fda preclinical genetic antibodies therapy pandemic covid-19 europeInternational
‘Excess Mortality Skyrocketed’: Tucker Carlson and Dr. Pierre Kory Unpack ‘Criminal’ COVID Response
‘Excess Mortality Skyrocketed’: Tucker Carlson and Dr. Pierre Kory Unpack ‘Criminal’ COVID Response
As the global pandemic unfolded, government-funded…

As the global pandemic unfolded, government-funded experimental vaccines were hastily developed for a virus which primarily killed the old and fat (and those with other obvious comorbidities), and an aggressive, global campaign to coerce billions into injecting them ensued.
Then there were the lockdowns - with some countries (New Zealand, for example) building internment camps for those who tested positive for Covid-19, and others such as China welding entire apartment buildings shut to trap people inside.
It was an egregious and unnecessary response to a virus that, while highly virulent, was survivable by the vast majority of the general population.
Oh, and the vaccines, which governments are still pushing, didn't work as advertised to the point where health officials changed the definition of "vaccine" multiple times.
Tucker Carlson recently sat down with Dr. Pierre Kory, a critical care specialist and vocal critic of vaccines. The two had a wide-ranging discussion, which included vaccine safety and efficacy, excess mortality, demographic impacts of the virus, big pharma, and the professional price Kory has paid for speaking out.
Keep reading below, or if you have roughly 50 minutes, watch it in its entirety for free on X:
Ep. 81 They’re still claiming the Covid vax is safe and effective. Yet somehow Dr. Pierre Kory treats hundreds of patients who’ve been badly injured by it. Why is no one in the public health establishment paying attention? pic.twitter.com/IekW4Brhoy
— Tucker Carlson (@TuckerCarlson) March 13, 2024
"Do we have any real sense of what the cost, the physical cost to the country and world has been of those vaccines?" Carlson asked, kicking off the interview.
"I do think we have some understanding of the cost. I mean, I think, you know, you're aware of the work of of Ed Dowd, who's put together a team and looked, analytically at a lot of the epidemiologic data," Kory replied. "I mean, time with that vaccination rollout is when all of the numbers started going sideways, the excess mortality started to skyrocket."
When asked "what kind of death toll are we looking at?", Kory responded "...in 2023 alone, in the first nine months, we had what's called an excess mortality of 158,000 Americans," adding "But this is in 2023. I mean, we've had Omicron now for two years, which is a mild variant. Not that many go to the hospital."
'Safe and Effective'
Tucker also asked Kory why the people who claimed the vaccine were "safe and effective" aren't being held criminally liable for abetting the "killing of all these Americans," to which Kory replied: "It’s my kind of belief, looking back, that [safe and effective] was a predetermined conclusion. There was no data to support that, but it was agreed upon that it would be presented as safe and effective."
Tucker Carlson Asks the Forbidden Question
— The Vigilant Fox ???? (@VigilantFox) March 14, 2024
He wants to know why the people who made the claim “safe and effective” aren’t being held to criminal liability for abetting the “killing of all these Americans.”
DR. PIERRE KORY: “It’s my kind of belief, looking back, that [safe and… pic.twitter.com/Icnge18Rtz
Carlson and Kory then discussed the different segments of the population that experienced vaccine side effects, with Kory noting an "explosion in dying in the youngest and healthiest sectors of society," adding "And why did the employed fare far worse than those that weren't? And this particularly white collar, white collar, more than gray collar, more than blue collar."
Kory also said that Big Pharma is 'terrified' of Vitamin D because it "threatens the disease model." As journalist The Vigilant Fox notes on X, "Vitamin D showed about a 60% effectiveness against the incidence of COVID-19 in randomized control trials," and "showed about 40-50% effectiveness in reducing the incidence of COVID-19 in observational studies."
Dr. Pierre Kory: Big Pharma is ‘TERRIFIED’ of Vitamin D
— The Vigilant Fox ???? (@VigilantFox) March 14, 2024
Why?
Because “It threatens the DISEASE MODEL.”
A new meta-analysis out of Italy, published in the journal, Nutrients, has unearthed some shocking data about Vitamin D.
Looking at data from 16 different studies and 1.26… pic.twitter.com/q5CsMqgVju
Professional costs
Kory - while risking professional suicide by speaking out, has undoubtedly helped save countless lives by advocating for alternate treatments such as Ivermectin.
Kory shared his own experiences of job loss and censorship, highlighting the challenges of advocating for a more nuanced understanding of vaccine safety in an environment often resistant to dissenting voices.
"I wrote a book called The War on Ivermectin and the the genesis of that book," he said, adding "Not only is my expertise on Ivermectin and my vast clinical experience, but and I tell the story before, but I got an email, during this journey from a guy named William B Grant, who's a professor out in California, and he wrote to me this email just one day, my life was going totally sideways because our protocols focused on Ivermectin. I was using a lot in my practice, as were tens of thousands of doctors around the world, to really good benefits. And I was getting attacked, hit jobs in the media, and he wrote me this email on and he said, Dear Dr. Kory, what they're doing to Ivermectin, they've been doing to vitamin D for decades..."
"And it's got five tactics. And these are the five tactics that all industries employ when science emerges, that's inconvenient to their interests. And so I'm just going to give you an example. Ivermectin science was extremely inconvenient to the interests of the pharmaceutical industrial complex. I mean, it threatened the vaccine campaign. It threatened vaccine hesitancy, which was public enemy number one. We know that, that everything, all the propaganda censorship was literally going after something called vaccine hesitancy."
Money makes the world go 'round
Carlson then hit on perhaps the most devious aspect of the relationship between drug companies and the medical establishment, and how special interests completely taint science to the point where public distrust of institutions has spiked in recent years.
"I think all of it starts at the level the medical journals," said Kory. "Because once you have something established in the medical journals as a, let's say, a proven fact or a generally accepted consensus, consensus comes out of the journals."
"I have dozens of rejection letters from investigators around the world who did good trials on ivermectin, tried to publish it. No thank you, no thank you, no thank you. And then the ones that do get in all purportedly prove that ivermectin didn't work," Kory continued.
"So and then when you look at the ones that actually got in and this is where like probably my biggest estrangement and why I don't recognize science and don't trust it anymore, is the trials that flew to publication in the top journals in the world were so brazenly manipulated and corrupted in the design and conduct in, many of us wrote about it. But they flew to publication, and then every time they were published, you saw these huge PR campaigns in the media. New York Times, Boston Globe, L.A. times, ivermectin doesn't work. Latest high quality, rigorous study says. I'm sitting here in my office watching these lies just ripple throughout the media sphere based on fraudulent studies published in the top journals. And that's that's that has changed. Now that's why I say I'm estranged and I don't know what to trust anymore."
Vaccine Injuries
Carlson asked Kory about his clinical experience with vaccine injuries.
"So how this is how I divide, this is just kind of my perception of vaccine injury is that when I use the term vaccine injury, I'm usually referring to what I call a single organ problem, like pericarditis, myocarditis, stroke, something like that. An autoimmune disease," he replied.
"What I specialize in my practice, is I treat patients with what we call a long Covid long vaxx. It's the same disease, just different triggers, right? One is triggered by Covid, the other one is triggered by the spike protein from the vaccine. Much more common is long vax. The only real differences between the two conditions is that the vaccinated are, on average, sicker and more disabled than the long Covids, with some pretty prominent exceptions to that."
Watch the entire interview above, and you can support Tucker Carlson's endeavors by joining the Tucker Carlson Network here...
International
Shakira’s net worth
After 12 albums, a tax evasion case, and now a towering bronze idol sculpted in her image, how much is Shakira worth more than 4 decades into her care…

Shakira’s considerable net worth is no surprise, given her massive popularity in Latin America, the U.S., and elsewhere.
In fact, the belly-dancing contralto queen is the second-wealthiest Latin-America-born pop singer of all time after Gloria Estefan. (Interestingly, Estefan actually helped a young Shakira translate her breakout album “Laundry Service” into English, hugely propelling her stateside success.)
Since releasing her first record at age 13, Shakira has spent decades recording albums in both Spanish and English and performing all over the world. Over the course of her 40+ year career, she helped thrust Latin pop music into the American mainstream, paving the way for the subsequent success of massively popular modern acts like Karol G and Bad Bunny.
In December 2023, a 21-foot-tall beachside bronze statue of the “Hips Don’t Lie” singer was unveiled in her Colombian hometown of Barranquilla, making her a permanent fixture in the city’s skyline and cementing her legacy as one of Latin America’s most influential entertainers.
After 12 albums, a plethora of film and television appearances, a highly publicized tax evasion case, and now a towering bronze idol sculpted in her image, how much is Shakira worth? What does her income look like? And how does she spend her money?
How much is Shakira worth?
In late 2023, Spanish sports and lifestyle publication Marca reported Shakira’s net worth at $400 million, citing Forbes as the figure’s source (although Forbes’ profile page for Shakira does not list a net worth — and didn’t when that article was published).
Most other sources list the singer’s wealth at an estimated $300 million, and almost all of these point to Celebrity Net Worth — a popular but dubious celebrity wealth estimation site — as the source for the figure.
A $300 million net worth would make Shakira the third-richest Latina pop star after Gloria Estefan ($500 million) and Jennifer Lopez ($400 million), and the second-richest Latin-America-born pop singer after Estefan (JLo is Puerto Rican but was born in New York).
Shakira’s income: How much does she make annually?
Entertainers like Shakira don’t have predictable paychecks like ordinary salaried professionals. Instead, annual take-home earnings vary quite a bit depending on each year’s album sales, royalties, film and television appearances, streaming revenue, and other sources of income. As one might expect, Shakira’s earnings have fluctuated quite a bit over the years.
From June 2018 to June 2019, for instance, Shakira was the 10th highest-earning female musician, grossing $35 million, according to Forbes. This wasn’t her first time gracing the top 10, though — back in 2012, she also landed the #10 spot, bringing in $20 million, according to Billboard.
In 2023, Billboard listed Shakira as the 16th-highest-grossing Latin artist of all time.

How much does Shakira make from her concerts and tours?
A large part of Shakira’s wealth comes from her world tours, during which she sometimes sells out massive stadiums and arenas full of passionate fans eager to see her dance and sing live.
According to a 2020 report by Pollstar, she sold over 2.7 million tickets across 190 shows that grossed over $189 million between 2000 and 2020. This landed her the 19th spot on a list of female musicians ranked by touring revenue during that period. In 2023, Billboard reported a more modest touring revenue figure of $108.1 million across 120 shows.
In 2003, Shakira reportedly generated over $4 million from a single show on Valentine’s Day at Foro Sol in Mexico City. 15 years later, in 2018, Shakira grossed around $76.5 million from her El Dorado World Tour, according to Touring Data.
Related: RuPaul's net worth: Everything to know about the cultural icon and force behind 'Drag Race'
How much has Shakira made from her album sales?
According to a 2023 profile in Variety, Shakira has sold over 100 million records throughout her career. “Laundry Service,” the pop icon’s fifth studio album, was her most successful, selling over 13 million copies worldwide, according to TheRichest.
Exactly how much money Shakira has taken home from her album sales is unclear, but in 2008, it was widely reported that she signed a 10-year contract with LiveNation to the tune of between $70 and $100 million to release her subsequent albums and manage her tours.

How much did Shakira make from her Super Bowl and World Cup performances?
Shakira co-wrote one of her biggest hits, “Waka Waka (This Time for Africa),” after FIFA selected her to create the official anthem for the 2010 World Cup in South Africa. She performed the song, along with several of her existing fan-favorite tracks, during the event’s opening ceremonies. TheThings reported in 2023 that the song generated $1.4 million in revenue, citing Popnable for the figure.
A decade later, 2020’s Superbowl halftime show featured Shakira and Jennifer Lopez as co-headliners with guest performances by Bad Bunny and J Balvin. The 14-minute performance was widely praised as a high-energy celebration of Latin music and dance, but as is typical for Super Bowl shows, neither Shakira nor JLo was compensated beyond expenses and production costs.
The exposure value that comes with performing in the Super Bowl Halftime Show, though, is significant. It is typically the most-watched television event in the U.S. each year, and in 2020, a 30-second Super Bowl ad spot cost between $5 and $6 million.
How much did Shakira make as a coach on “The Voice?”
Shakira served as a team coach on the popular singing competition program “The Voice” during the show’s fourth and sixth seasons. On the show, celebrity musicians coach up-and-coming amateurs in a team-based competition that eventually results in a single winner. In 2012, The Hollywood Reporter wrote that Shakira’s salary as a coach on “The Voice” was $12 million.
Related: John Cena's net worth: The wrestler-turned-actor's investments, businesses, and more
How does Shakira spend her money?
Shakira doesn’t just make a lot of money — she spends it, too. Like many wealthy entertainers, she’s purchased her share of luxuries, but Barranquilla’s barefoot belly dancer is also a prolific philanthropist, having donated tens of millions to charitable causes throughout her career.
Private island
Back in 2006, she teamed up with Roger Waters of Pink Floyd fame and Spanish singer Alejandro Sanz to purchase Bonds Cay, a 550-acre island in the Bahamas, which was listed for $16 million at the time.
Along with her two partners in the purchase, Shakira planned to develop the island to feature housing, hotels, and an artists’ retreat designed to host a revolving cast of artists-in-residence. This plan didn’t come to fruition, though, and as of this article’s last update, the island was once again for sale on Vladi Private Islands.
Real estate and vehicles
Like most wealthy celebs, Shakira’s portfolio of high-end playthings also features an array of luxury properties and vehicles, including a home in Barcelona, a villa in Cyprus, a Miami mansion, and a rotating cast of Mercedes-Benz vehicles.
Philanthropy and charity
Shakira doesn’t just spend her massive wealth on herself; the “Queen of Latin Music” is also a dedicated philanthropist and regularly donates portions of her earnings to the Fundación Pies Descalzos, or “Barefoot Foundation,” a charity she founded in 1997 to “improve the education and social development of children in Colombia, which has suffered decades of conflict.” The foundation focuses on providing meals for children and building and improving educational infrastructure in Shakira’s hometown of Barranquilla as well as four other Colombian communities.
In addition to her efforts with the Fundación Pies Descalzos, Shakira has made a number of other notable donations over the years. In 2007, she diverted a whopping $40 million of her wealth to help rebuild community infrastructure in Peru and Nicaragua in the wake of a devastating 8.0 magnitude earthquake. Later, during the COVID-19 pandemic in 2020, Shakira donated a large supply of N95 masks for healthcare workers and ventilators for hospital patients to her hometown of Barranquilla.
Back in 2010, the UN honored Shakira with a medal to recognize her dedication to social justice, at which time the Director General of the International Labour Organization described her as a “true ambassador for children and young people.”

Shakira’s tax fraud scandal: How much did she pay?
In 2018, prosecutors in Spain initiated a tax evasion case against Shakira, alleging she lived primarily in Spain from 2012 to 2014 and therefore failed to pay around $14.4 million in taxes to the Spanish government. Spanish law requires anyone who is “domiciled” (i.e., living primarily) in Spain for more than half of the year to pay income taxes.
During the period in question, Shakira listed the Bahamas as her primary residence but did spend some time in Spain, as she was dating Gerard Piqué, a professional footballer and Spanish citizen. The couple’s first son, Milan, was also born in Barcelona during this period.
Shakira maintained that she spent far fewer than 183 days per year in Spain during each of the years in question. In an interview with Elle Magazine, the pop star opined that “Spanish tax authorities saw that I was dating a Spanish citizen and started to salivate. It's clear they wanted to go after that money no matter what."
Prosecutors in the case sought a fine of almost $26 million and a possible eight-year prison stint, but in November of 2023, Shakira took a deal to close the case, accepting a fine of around $8 million and a three-year suspended sentence to avoid going to trial. In reference to her decision to take the deal, Shakira stated, "While I was determined to defend my innocence in a trial that my lawyers were confident would have ruled in my favour [had the trial proceeded], I have made the decision to finally resolve this matter with the best interest of my kids at heart who do not want to see their mom sacrifice her personal well-being in this fight."
How much did the Shakira statue in Barranquilla cost?
In late 2023, a 21-foot-tall bronze likeness of Shakira was unveiled on a waterfront promenade in Barranquilla. The city’s then-mayor, Jaime Pumarejo, commissioned Colombian sculptor Yino Márquez to create the statue of the city’s treasured pop icon, along with a sculpture of the city’s coat of arms.
According to the New York Times, the two sculptures cost the city the equivalent of around $180,000. A plaque at the statue’s base reads, “A heart that composes, hips that don’t lie, an unmatched talent, a voice that moves the masses and bare feet that march for the good of children and humanity.”
Related: Taylor Swift net worth: The most successful entertainer joins the billionaire's club
bonds pandemic covid-19 real estate africa mexico spainInternational
Delta Air Lines adds a new route travelers have been asking for
The new Delta seasonal flight to the popular destination will run daily on a Boeing 767-300.

Those who have tried to book a flight from North America to Europe in the summer of 2023 know just how high travel demand to the continent has spiked.
At 2.93 billion, visitors to the countries making up the European Union had finally reached pre-pandemic levels last year while North Americans in particular were booking trips to both large metropolises such as Paris and Milan as well as smaller cities growing increasingly popular among tourists.
Related: A popular European city is introducing the highest 'tourist tax' yet
As a result, U.S.-based airlines have been re-evaluating their networks to add more direct routes to smaller European destinations that most travelers would have previously needed to reach by train or transfer flight with a local airline.
Shutterstock
Delta Air Lines: ‘Glad to offer customers increased choice…’
By the end of March, Delta Air Lines (DAL) will be restarting its route between New York’s JFK and Marco Polo International Airport in Venice as well as launching two new flights to Venice from Atlanta. One will start running this month while the other will be added during peak demand in the summer.
More Travel:
- A new travel term is taking over the internet (and reaching airlines and hotels)
- The 10 best airline stocks to buy now
- Airlines see a new kind of traveler at the front of the plane
“As one of the most beautiful cities in the world, Venice is hugely popular with U.S. travelers, and our flights bring valuable tourism and trade opportunities to the city and the region as well as unrivalled opportunities for Venetians looking to explore destinations across the Americas,” Delta’s SVP for Europe Matteo Curcio said in a statement. “We’re glad to offer customers increased choice this summer with flights from New York and additional service from Atlanta.”
The JFK-Venice flight will run on a Boeing 767-300 (BA) and have 216 seats including higher classes such as Delta One, Delta Premium Select and Delta Comfort Plus.
Delta offers these features on the new flight
Both the New York and Atlanta flights are seasonal routes that will be pulled out of service in October. Both will run daily while the first route will depart New York at 8:55 p.m. and arrive in Venice at 10:15 a.m. local time on the way there, while leaving Venice at 12:15 p.m. to arrive at JFK at 5:05 p.m. on the way back.
According to Delta, this will bring its service to 17 flights from different U.S. cities to Venice during the peak summer period. As with most Delta flights at this point, passengers in all fare classes will have access to free Wi-Fi during the flight.
Those flying in Delta’s highest class or with access through airline status or a credit card will also be able to use the new Delta lounge that is part of the airline’s $12 billion terminal renovation and is slated to open to travelers in the coming months. The space will take up more than 40,000 square feet and have an outdoor terrace.
“Delta One customers can stretch out in a lie-flat seat and enjoy premium amenities like plush bedding made from recycled plastic bottles, more beverage options, and a seasonal chef-curated four-course meal,” Delta said of the new route. “[…] All customers can enjoy a wide selection of in-flight entertainment options and stay connected with Wi-Fi and enjoy free mobile messaging.”
stocks pandemic european europe-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International7 days ago
International7 days agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International7 days ago
International7 days agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges






















