Government
After 28 Days On Ventilator, Family Loses Legal Battle To Try Ivermectin, Other Alternative Treatments, For Dying Father
After 28 Days On Ventilator, Family Loses Legal Battle To Try Ivermectin, Other Alternative Treatments, For Dying Father
Authored by Nanette Holt via The Epoch Times,
A Florida family fighting to give their loved one on a ventilator alternat
Authored by Nanette Holt via The Epoch Times,
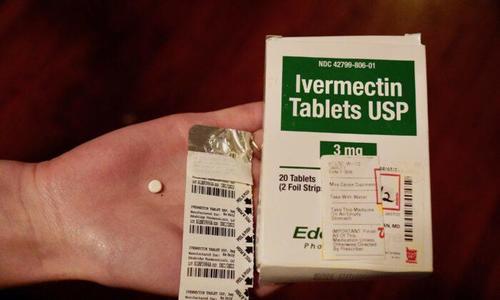
A Florida family fighting to give their loved one on a ventilator alternative treatments for COVID-19 have lost another battle—this time in Florida’s First District Court of Appeal.
The wife and son of Daniel Pisano first squared off against Mayo Clinic Florida at an emergency hearing on Dec. 30 in Florida’s Fourth Judicial Circuit. Before that, they’d been begging the hospital to allow them to try treating Pisano—who’s been on a ventilator now for 28 days—with the controversial drug ivermectin, along with a mix of other drugs and supplements, part of a protocol recommended by the Front Line COVID-19 Critical Care Alliance (FLCCC).
The family’s request for an emergency injunction to force the Mayo Clinic to allow treatments recommended by an outside doctor was denied by Judge Marianne Aho. They appealed the decision.
On Jan. 14, Aho’s decision was upheld by Florida’s First District Court of Appeal. The three-judge panel deciding the case included Judge Thomas “Bo” Winokur, appointed by then-Gov. Rick Scott in 2015; Judge M. Kemmerly Thomas, appointed in 2016 by Scott; and Judge Robert E. Long, Jr., appointed in 2020, by Gov. Ron DeSantis.
“An opinion of this Court explaining its reasoning will follow,” the judges stated in the order they issued.
“So we wait to see what that looks like, unless it takes too long,” said Jeff Childers, an attorney for the family.
Seventy-year-old Daniel Pisano doesn’t have unlimited time, says Eduardo Balbona, M.D., an independent doctor in Jacksonville who’s been advising the family since they reached out to him while researching other treatments that could potentially help their loved one.
Daniel and Claudia Pisano moved to Florida and bought a homesite to be 20 minutes from their only two grandchildren. (Photo courtesy of Chris Pisano)
Balbona, who has been monitoring Pisano’s treatment at the Mayo Clinic through an online portal, testified on behalf of the Pisano family in the first hearing.
The Mayo Clinic has argued that the treatment plan doesn’t fit with the hospital’s standard protocol for treating COVID-19 patients and they don’t know what the effects of following Balbona’s recommendations would be. The hospital has told the family that Pisano has a less-than-five percent chance of survival, and all that’s left to do is wait and see if he recovers on the ventilator. The Mayo Clinic has not responded to requests for comment.
The family has begged the Mayo Clinic to simply step aside and let Balbona try what he thinks could work. But the Mayo Clinic doesn’t allow outside doctors to treat patients.
Since media reports mentioned his involvement in the case, particularly his confidence in recommending ivermectin, Balbona has faced a mix of hate-filled criticism and desperate cries for help.
He says he’s used ivermectin along with the rest of the FLCCC protocol successfully with minor modifications, on “dozens and dozens” of seriously ill patients suffering the effects of COVID-19. Some of those patients have come to him from as far away as California.
He’s not alone in his belief in ivermectin and the mix of drugs and supplements he’s suggesting. Different health care professionals across the country have spoken out over the past two years about the efficacy of using ivermectin and the FLCCC protocol to treat COVID-19.
The drug has been used for 40 years and won a Nobel Prize for its creator. While ivermectin is most often used to prevent or kill parasites in animals, it has also been widely and successfully used for years to treat parasites and viruses in humans in the United States and other countries. There is an ever-growing list of peer-reviewed studies showing the drug’s efficacy in treating COVID-19.
The U.S. Food and Drug Administration (FDA) indicates there are ongoing clinical trials investigating the use of the drug in the treatment of COVID-19 on a webpage warning people not to self-medicate with ivermectin. The FDA published a tweet in August mocking those who do. And some politicians and media outlets have railed relentlessly against those claiming ivermectin could be an effective and inexpensive way to combat COVID-19.
The U.S. Food and Drug Administration (FDA) shared this tweet on Aug. 21, 2021, mocking the use of the drug ivermectin in the treatment of COVID-19. (Photo courtesy of FDA via Twitter)
“You should be embarrassed to practice medicine, to sue the Mayo Clinic to get horse medicine to a human being, because of Internet garbage,” one person seethed on a voicemail at Balbona’s office after his court testimony was mentioned in an Epoch Times article.
“Your license should be revoked, you worthless piece of garbage. You are killing people, not helping them, and to harass the Mayo Clinic, because you are not good enough to be their doctor is disgusting. Disgusting. You and doctors like you should all be banned from society. Shame on you. Disgusting. Goodbye and good riddance. I hope you get COVID. Goodbye.”
Balbona says he deletes messages like that and pushes on with his treatment of patients.
It’s “just the intolerance and hatred that takes me by surprise,” he said, about his office communications now getting “flooded by hate.”
Eduardo Balbona, M.D., completed specialty training in internal medicine at the National Naval Medical Center and served as a physician at the U.S. Capitol, caring for senators, congressmen and Supreme Court justices. (Photo courtesy of Eduardo Balbona, M.D.)
“Everything I do treating COVID is directed at lowering the inflammatory response, which is out of control, and improving blood flow to the lungs, and avoiding the complications of clots,” he said.
“Perhaps the biggest change I’ve made from protocols in the hospital and with FLCCC is increasing the dose of dexamethasone. The dose of dexamethasone in FLCCC is relatively low at 6 mg, and I generally increase that to 18 mg daily in more serious cases. That’s a logic change, and I realize the study support is at 6 mg.”
“There’s a reason for every medicine and everything I do treating COVID with my protocol. I have to be able to defend it since I know it will be attacked. Crazy world we’re in.”
Christie DeTrude, of Switzerland, Florida, feels certain that Balbona’s recommendations saved her husband, Dewey. He had just retired last spring at 59 after a long career as a pipe-fitter. At 200 pounds and 6-feet-tall, he was in the peak of health, with strong “country muscles after a lifetime of turning a wrench,” she said.
Dewey and Christie DeTrude on vacation in Hawaii, before he fell ill with COVID-19. (Courtesy of the DeTrude Family)
When he sought treatment for COVID-19 at an urgent-care clinic in July, he was prescribed ivermectin by a doctor there.
“But what we didn’t know at the time was, it wasn’t a high enough dose, because it’s supposed to be weight-based,” Christie DeTrude said. “Theirs was a very low dose, and they discontinued it after five days and said that it would be damaging to his liver and kidneys if they continued, which isn’t true.”
On his eighth day of illness, he had developed pneumonia, and the urgent-care clinic told him to go to the hospital for treatment with convalescent plasma and oxygen. The referring doctor promised he wouldn’t be admitted, Christie DeTrude said.
When she dropped him off at the Mayo Clinic Florida emergency room, she was told to come back and pick him up in 4-5 hours.
“Once he got to Mayo, they just completely took over, and there was no informed consent,” DeTrude said. “There was no giving him information and letting us make a decision. They made all of his decisions for him, and they follow a standard protocol.”
“There were no choices, there was no discussion…they just kept upping the oxygen,” DeTrude said.
The Mayo Clinic did not return requests for comment by The Epoch Times about DeTrude’s case, Pisano’s case, or COVID-19 treatment protocols, in general.
DeTrude said that eventually, her husband had become so weak, he couldn’t get out of the hospital bed. She felt that the hospital’s treatments weren’t working. She wanted to take him home. The hospital wouldn’t agree to discharge him and didn’t allow her to visit, she said.
Dewey DeTrude’s wife hired an attorney to help her get her husband out of the intensive care unit at Mayo Clinic Florida, so he could be treated at home with ivermectin. DeTrude, shown here on Aug. 3, 2021, spent 46 days in the hospital. (Courtesy of the DeTrude Family)
Days passed. Then, weeks. She says that she could tell from their phone calls that her husband was getting weaker. His 60th birthday came and went. And still, she says the hospital wouldn’t let her visit.
“I was able to get a Catholic priest to come give him Last Rites, and the priest said that my husband’s mental state was like that of a prisoner of war, that he was definitely suffering trauma from the isolation from family, from his faith, from not seeing the sun. He’d lost 35 pounds,” she said.
Part of the problem was that she wasn’t allowed to bring him vegan meals, she said.
“A lot of the food, my husband wasn’t interested in. And when you’re on oxygen, it does affect your appetite, and he needed assistance eating, but they wouldn’t let me be that person,” she said.
After 18 days, Christie DeTrude hired an attorney to help her push the hospital to stabilize her husband so she could take him home. Meanwhile, she searched for an outside doctor who could help.
With that aim, she attended a medical freedom rally in Jacksonville in August, hoping to find something or someone who could advise her. Several doctors spoke about alternative treatments for COVID-19 that hospitals weren’t using, including ivermectin.
The next day, she called them all. Only Dr. Balbona came to the phone to speak with her, she said.
At Christie DeTrude’s request, Balbona promised the hospital that he’d take over her husband’s care. He ordered oxygen, medication, and home-health assistance for the family, she said.
As she waited for Mayo doctors to agree to discharge him, Christie DeTrude prayed every day that her husband could hang on a little longer.
After 46 days at Mayo Clinic, Dewey DeTrude finally was discharged and immediately started following Dr. Balbona’s instructions, taking ivermectin, fluvoxamine to prevent blood clots, and propranolol to treat anxiety and post-traumatic stress disorder from his hospital stay. He also took Vitamin C, Vitamin D, and zinc. He ate healthy food and spent time in the sunshine. Within days, it was clear her husband was on the mend, Christie DeTrude said.
Now, four months later, “he’s working part-time, going to the gym,” she said. “He’s completed physical therapy and working on rebuilding his stamina and lung capacity. And if it weren’t for Dr. Balbona, I’m quite sure he would have died in the hospital.”
Gene Bennett, a 77-year-old retired field engineer for IBM, tells a similar story.
He was enjoying life in Bryceville, Florida helping his son clear five acres of land for a homesite when COVID-19 struck in January 2021.
An ambulance transported him to Ascension St. Vincent’s Riverside Hospital in Jacksonville, where he was treated with remdesivir.
“They had to keep getting my oxygen higher and higher,” Bennett said. “I was finally up to the point of seven liters per minute, which is almost pure oxygen. And I knew that I wasn’t getting better. I could tell I was getting weaker and weaker. So when the doctor made his rounds on the Monday morning, I said, ‘This is my last day of remdesivir treatment and I know that I’m not improving. What’s our next step?’
“He looked at me and very calmly said, ‘Mr. Bennett, we don’t have a next step.’ He said, ‘We have done all for you that we can do. There’s nothing else we can do for you.’”
Gene Bennett insisted on leaving the hospital, instead of going on a ventilator. (Courtesy of Jane Bennett)
Overnight, Bennett thought a lot about the conversation. The next day, he asked the doctor, “Are you serious? There’s nothing else that this hospital can do for me?”
“He said, ‘No, sir. The next step is for you to go on a ventilator.’”
“Well, I’m not going to do that,” Bennett recalls saying. “I want to be released from this hospital.”
He quickly learned that was no longer a decision he could make for himself.
Ascension St. Vincent’s Riverside Hospital did not respond to a request for comment.
“They weren’t going to release me because I was on a high level of oxygen,” he told The Epoch Times. “So finally, after I raised hell with them, to put it mildly, all day, my son picked me up” that evening.
The next morning, Bennett’s wife drove him to Dr. Balbona, his physician for many years. Balbona came out to the parking lot of his office to help him out of the car.
“I could barely walk with a walker without assistance — that’s how bad off I was,” Bennett said. He says Balbona told him, ” You have the most severe case of COVID that I have seen. But I have a medicine I have been using and I’ve had great success with it.”
Bennett needed no convincing.
“What is it? I’ll take it,” Bennett recalls saying. “I know I’m dying. I just feel it.”
“He told me and my wife, ‘Most people that have COVID as severe as you do not survive. We’re behind the curve, but we’re going to try to get you over the hump. The medicine I’d like to prescribe for you is normally a heartworm medicine for dogs—that’s the most common use.’
“He said, ‘They use it all over the world. It’s been around for 40 years, and it’s dirt cheap, but very effective.’
“He said, ‘I would never, ever give a patient a medicine that I thought would be harmful to them.’ And I totally believed, and just accepted the fact he was doing what he thinks was right.
“I thought, I don’t have any options. I know if I don’t take something to stop this, it’s going to kill me.”
They picked up a $30 supply of ivermectin from a drug store that day. Bennett was so weak, he could barely feed himself. His wife and son later told him that they thought he was going to die.
But after five days on what Dr. Balbona prescribed, including Vitamin C, Vitamin D, zinc, steroids, and a diuretic to get fluid off his lungs, he started to improve.
“I’m a firm believer and I’d swear on the Bible, had I not been prescribed ivermectin, I would have died. Had I not stepped out of St. Vincent’s and checked myself out and gone to him and got the ivermectin, I wouldn’t be talking to you today. It saved my life. And for how much money? Thirty dollars!”
He has since read a lot of research about the efficacy of ivermectin in the treatment of COVID-19.
Gene Bennett refused to go on a ventilator when he was seriously ill with COVID-19. After leaving the hospital, his doctor treated him with ivermectin. He made a full recovery. (Courtesy of Jane Bennett)
“I can’t tell you if it is 100 percent effective for everyone, but I can tell you it was for me. I personally cannot understand why the government balks at giving these treatments. Why don’t they make the announcement that it’s available and let it be an individual’s choice?”
Ivermectin has been approved for the treatment of COVID-19 in all or part of 22 countries.
Over the past year, Bennett’s gotten back to full health, almost, regaining about half of the 45 pounds he lost while he was ill.
His wife’s brother died in early January of COVID-19. They begged the hospital to try ivermectin. The hospital declined.
His daughter-in-law’s mother died of COVID-19, too, in a Jacksonville Beach hospital, after the family begged to try ivermectin, and the hospital refused, Bennett said.
An FDA spokeswoman said she would provide the number of reports of patients who had problems after self-medicating with ivermectin. Three days later, that information had not been provided to The Epoch Times.
The FDA Office of Media Affairs said a formal request under the Freedom of Information Act (FOIA) would be required to obtain details about when ivermectin might be approved for use in treating COVID-19, and about bonafide injuries to people who’ve used ivermectin to treat the illness.
“The most effective ways to limit the spread of COVID-19 include getting a COVID-19 vaccine when it is available to you and following current CDC guidance,” the FDA’s website advises.
The Epoch Times spoke to a dozen people who have used ivermectin formulated for humans to treat COVID-19 at home. Most obtained prescriptions for the drug through online medical services. None reported having any side effects, even those who admitted to using ivermectin formulated for animals.
Government
Low Iron Levels In Blood Could Trigger Long COVID: Study
Low Iron Levels In Blood Could Trigger Long COVID: Study
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
People with inadequate…
Authored by Amie Dahnke via The Epoch Times (emphasis ours),
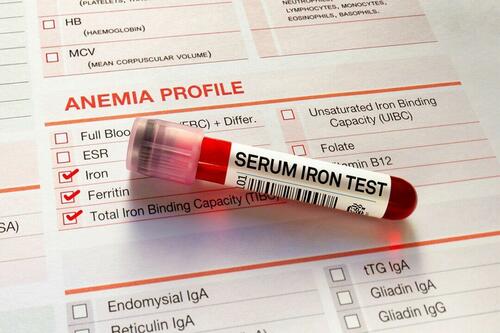
People with inadequate iron levels in their blood due to a COVID-19 infection could be at greater risk of long COVID.
A new study indicates that problems with iron levels in the bloodstream likely trigger chronic inflammation and other conditions associated with the post-COVID phenomenon. The findings, published on March 1 in Nature Immunology, could offer new ways to treat or prevent the condition.
Long COVID Patients Have Low Iron Levels
Researchers at the University of Cambridge pinpointed low iron as a potential link to long-COVID symptoms thanks to a study they initiated shortly after the start of the pandemic. They recruited people who tested positive for the virus to provide blood samples for analysis over a year, which allowed the researchers to look for post-infection changes in the blood. The researchers looked at 214 samples and found that 45 percent of patients reported symptoms of long COVID that lasted between three and 10 months.
In analyzing the blood samples, the research team noticed that people experiencing long COVID had low iron levels, contributing to anemia and low red blood cell production, just two weeks after they were diagnosed with COVID-19. This was true for patients regardless of age, sex, or the initial severity of their infection.
According to one of the study co-authors, the removal of iron from the bloodstream is a natural process and defense mechanism of the body.
But it can jeopardize a person’s recovery.
“When the body has an infection, it responds by removing iron from the bloodstream. This protects us from potentially lethal bacteria that capture the iron in the bloodstream and grow rapidly. It’s an evolutionary response that redistributes iron in the body, and the blood plasma becomes an iron desert,” University of Oxford professor Hal Drakesmith said in a press release. “However, if this goes on for a long time, there is less iron for red blood cells, so oxygen is transported less efficiently affecting metabolism and energy production, and for white blood cells, which need iron to work properly. The protective mechanism ends up becoming a problem.”
The research team believes that consistently low iron levels could explain why individuals with long COVID continue to experience fatigue and difficulty exercising. As such, the researchers suggested iron supplementation to help regulate and prevent the often debilitating symptoms associated with long COVID.
“It isn’t necessarily the case that individuals don’t have enough iron in their body, it’s just that it’s trapped in the wrong place,” Aimee Hanson, a postdoctoral researcher at the University of Cambridge who worked on the study, said in the press release. “What we need is a way to remobilize the iron and pull it back into the bloodstream, where it becomes more useful to the red blood cells.”
The research team pointed out that iron supplementation isn’t always straightforward. Achieving the right level of iron varies from person to person. Too much iron can cause stomach issues, ranging from constipation, nausea, and abdominal pain to gastritis and gastric lesions.
1 in 5 Still Affected by Long COVID
COVID-19 has affected nearly 40 percent of Americans, with one in five of those still suffering from symptoms of long COVID, according to the U.S. Centers for Disease Control and Prevention (CDC). Long COVID is marked by health issues that continue at least four weeks after an individual was initially diagnosed with COVID-19. Symptoms can last for days, weeks, months, or years and may include fatigue, cough or chest pain, headache, brain fog, depression or anxiety, digestive issues, and joint or muscle pain.
Government
Walmart joins Costco in sharing key pricing news
The massive retailers have both shared information that some retailers keep very close to the vest.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trumpGovernment
Walmart has really good news for shoppers (and Joe Biden)
The giant retailer joins Costco in making a statement that has political overtones, even if that’s not the intent.

As we head toward a presidential election, the presumed candidates for both parties will look for issues that rally undecided voters.
The economy will be a key issue, with Democrats pointing to job creation and lowering prices while Republicans will cite the layoffs at Big Tech companies, high housing prices, and of course, sticky inflation.
The covid pandemic created a perfect storm for inflation and higher prices. It became harder to get many items because people getting sick slowed down, or even stopped, production at some factories.
Related: Popular mall retailer shuts down abruptly after bankruptcy filing
It was also a period where demand increased while shipping, trucking and delivery systems were all strained or thrown out of whack. The combination led to product shortages and higher prices.
You might have gone to the grocery store and not been able to buy your favorite paper towel brand or find toilet paper at all. That happened partly because of the supply chain and partly due to increased demand, but at the end of the day, it led to higher prices, which some consumers blamed on President Joe Biden's administration.
Biden, of course, was blamed for the price increases, but as inflation has dropped and grocery prices have fallen, few companies have been up front about it. That's probably not a political choice in most cases. Instead, some companies have chosen to lower prices more slowly than they raised them.
However, two major retailers, Walmart (WMT) and Costco, have been very honest about inflation. Walmart Chief Executive Doug McMillon's most recent comments validate what Biden's administration has been saying about the state of the economy. And they contrast with the economic picture being painted by Republicans who support their presumptive nominee, Donald Trump.
Image source: Joe Raedle/Getty Images
Walmart sees lower prices
McMillon does not talk about lower prices to make a political statement. He's communicating with customers and potential customers through the analysts who cover the company's quarterly-earnings calls.
During Walmart's fiscal-fourth-quarter-earnings call, McMillon was clear that prices are going down.
"I'm excited about the omnichannel net promoter score trends the team is driving. Across countries, we continue to see a customer that's resilient but looking for value. As always, we're working hard to deliver that for them, including through our rollbacks on food pricing in Walmart U.S. Those were up significantly in Q4 versus last year, following a big increase in Q3," he said.
He was specific about where the chain has seen prices go down.
"Our general merchandise prices are lower than a year ago and even two years ago in some categories, which means our customers are finding value in areas like apparel and hard lines," he said. "In food, prices are lower than a year ago in places like eggs, apples, and deli snacks, but higher in other places like asparagus and blackberries."
McMillon said that in other areas prices were still up but have been falling.
"Dry grocery and consumables categories like paper goods and cleaning supplies are up mid-single digits versus last year and high teens versus two years ago. Private-brand penetration is up in many of the countries where we operate, including the United States," he said.
Costco sees almost no inflation impact
McMillon avoided the word inflation in his comments. Costco (COST) Chief Financial Officer Richard Galanti, who steps down on March 15, has been very transparent on the topic.
The CFO commented on inflation during his company's fiscal-first-quarter-earnings call.
"Most recently, in the last fourth-quarter discussion, we had estimated that year-over-year inflation was in the 1% to 2% range. Our estimate for the quarter just ended, that inflation was in the 0% to 1% range," he said.
Galanti made clear that inflation (and even deflation) varied by category.
"A bigger deflation in some big and bulky items like furniture sets due to lower freight costs year over year, as well as on things like domestics, bulky lower-priced items, again, where the freight cost is significant. Some deflationary items were as much as 20% to 30% and, again, mostly freight-related," he added.
bankruptcy pandemic trump-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 Uncategorized1 month ago
Uncategorized1 month agoCathie Wood sells a major tech stock (again)
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized2 weeks ago
Uncategorized2 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoIndustrial Production Decreased 0.1% in January
-

 International1 day ago
International1 day agoWalmart launches clever answer to Target’s new membership program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoGOP Efforts To Shore Up Election Security In Swing States Face Challenges




























