Government
4 Top Biotech Stocks To Watch This Week
Check out these biotech stocks that could prove to be valuable additions to your portfolio.
The post 4 Top Biotech Stocks To Watch This Week appeared first on Stock Market News, Quotes, Charts and Financial Information | StockMarket.com.

Are These The Best Biotech Stocks To Buy Right Now?
As we begin another week of trading, the stock market continues to break new highs. In light of this, biotech stocks continue to draw traction among investors. After all, this is an industry that constantly has new developments that would have a significant impact on the health care system. For instance, we saw Pfizer (NYSE: PFE) and Merck (NYSE: MRK) breaking through with the development of antiviral pills against COVID-19. Both companies have shown promising efficacy in trials of adults with COVID-19 who are at high risk of serious illness. Pfizer posted an efficacy of 89% while Merck’s was around 50%. Naturally, these new developments could be a potential game-changer in fighting the pandemic.
In addition, companies dealing with Alzheimer’s disease have also been under the spotlight lately. AC Immune’s (NASDAQ: ACIU) CEO Prof. Andrea Pfeifer expects the number of families that will be affected by Alzheimer’s could nearly triple by 2050 to 150 million. Thus, any new developments in the neurodegenerative disease space would be vital. With all said and done, the biotech industry rarely lacks exciting developments that would pique the interest of investors. With that in mind, here are four of the top biotech stocks in the stock market this week.
Best Biotech Stocks To Watch This Week
- Cassava Sciences Inc (NASDAQ: SAVA)
- Regeneron Pharmaceuticals Inc (NASDAQ: REGN)
- Novo Nordisk A/S (NYSE: NVO)
- Corcept Therapeutics Incorporated (NASDAQ: CORT)
Cassava Sciences
Firstly, we have the clinical-stage biotech company, Cassava Sciences. Essentially, it focuses on developing drugs for the treatment of neurodegenerative diseases, such as Alzheimer’s disease. Its products include Simufilam and SavaDx. SAVA stock has risen by more than 110% over the past week.

This is likely due to investors responding positively to a new development from the Journal of Neuroscience. Last week, the company was informed that there was no evidence of data manipulation in an article it published in July 2012. The article describes a new approach to treating Alzheimer’s disease. “I’ve never doubted the integrity of our people or science,” said Remi Barbier, President & CEO. “We remain focused on conducting a Phase 3 clinical program of Simufilam in people with Alzheimer’s disease. It’s an important endeavor, notwithstanding pundits who may be louder than they are learned. We’ll stay the course until our job is done.”
Well, it started with allegations of data manipulation that caused the company stock to dip in August. One of many claims was altered test images called Western blots. These allegations had such a big impact because the paper was foundational to Simufilam. So, now that these claims have been cleared, the worst could now be behind the company. With that in mind, would you add SAVA stock to your watchlist?
[Read More] Best Lithium Battery Stocks To Buy Now? 4 To Know
Regeneron Pharmaceuticals
Another top company in the biotech space right now would be Regeneron Pharmaceuticals. Put simply, the company specializes in medicines for the treatment of serious diseases. Also, it engages in the research, and development of monoclonal antibodies, which consists of Dupixent, Kevzara, and itepekimab. REGN stock has climbed more than 25% since the start of the year.

Earlier this month, the company reported its third-quarter earnings. Its revenue increased by 51% to $3.45 billion. Meanwhile, its GAAP net income was $1.63 billion, an increase of 94% year-over-year. The company says that it enjoyed strong growth in its core business this quarter. With EYLEA and Dupixent reaching more patients than ever, Regeneron appears to be firing on all cylinders to end the year on a high.
Furthermore, there are new additional positive results from a Phase 3 trial that assesses the use of a single dose of investigational REGEN-COV to prevent COVID-19 in uninfected individuals. Now, it shows that it reduces the risk of contracting COVID-19 by 81.6% during the pre-specified follow-up period (months 2-8), maintaining the 81.4% risk reduction previously reported during month 1. So, these results demonstrate that it has the potential to provide long-lasting immunity to those who do not respond to COVID-19 vaccines. Given these exciting developments, would you consider REGN stock as a top biotech stock to watch?
[Read More] Top Reddit Stocks To Buy Right Now? 5 For Your Late 2021 Watchlist
Novo Nordisk
Novo Nordisk is a global healthcare company that engages in diabetes care. The company also dwells in the discovery, development, manufacturing, and marketing of pharmaceutical products. Novo’s diabetes and obesity care segment covers insulin, GLP-1, other protein-related products, such as glucagon, protein-related delivery systems and needles, and oral anti-diabetic drugs.

Last Friday, the company presented results from the STEP 5 phase 3b trial at the ObesityWeek 2021 interactive congress. It demonstrated that adults treated with Wegovy achieved significant and sustained weight loss over the two-year study period. In most cases, people with obesity will often lose track and regain the weight that was previously lost. So, this result would give the company more confidence in better treating obesity as a chronic disease.
It is also noteworthy that Novo also announced a share repurchase program for an amount of up to $576 million. The purpose of the program is to reduce the company’s share capital and to meet obligations arising from share-based incentive programs. Besides that, companies usually initiate a share repurchase program when it feels that the company stock is undervalued. Given these considerations, would NVO stock be worth investing in right now?
[Read More] 5 Metaverse Stocks To Watch In November 2021
Corcept Therapeutics
To sum up the list, we will be looking at Corcept Therapeutics. In detail, the company engages in the discovery and development of drugs to treat severe metabolic, oncologic, and psychiatric disorders. It does so by modulating the effects of the steroid hormone cortisol. Impressively, CORT stock has risen more than 25% over the past month.

Today, the company started a tender offer to repurchase up to 10 million shares. The share buyback comes close on the heels of a better than expected third-quarter earnings posted last week. For the quarter, the company’s revenue came in 11% higher year over year to $96.1 million. Also, its GAAP diluted net income was $0.24 per share for the quarter. Corcept also ended the quarter with $495.2 million in cash and investments.
The company says that as the pandemic conditions continue to recede, it expects its growth to continue as physicians are seeing their patients more frequently and can diagnose and treat them with Cushing’s Syndrome. Cushing’s Syndrome is a disorder that occurs when the body makes too much of the hormone cortisol over a long period. Accordingly, enrollment continues for its Phase 3 GRACE trial of relacorilant as a treatment for patients with Cushing’s syndrome and it expects a new drug application submission by the second quarter of 2023. All things considered, will you be on the lookout for CORT stock?
The post 4 Top Biotech Stocks To Watch This Week appeared first on Stock Market News, Quotes, Charts and Financial Information | StockMarket.com.
nasdaq stocks pandemic covid-19 congress treatment antibodies monoclonal antibodiesInternational
Fuel poverty in England is probably 2.5 times higher than government statistics show
The top 40% most energy efficient homes aren’t counted as being in fuel poverty, no matter what their bills or income are.


The cap set on how much UK energy suppliers can charge for domestic gas and electricity is set to fall by 15% from April 1 2024. Despite this, prices remain shockingly high. The average household energy bill in 2023 was £2,592 a year, dwarfing the pre-pandemic average of £1,308 in 2019.
The term “fuel poverty” refers to a household’s ability to afford the energy required to maintain adequate warmth and the use of other essential appliances. Quite how it is measured varies from country to country. In England, the government uses what is known as the low income low energy efficiency (Lilee) indicator.
Since energy costs started rising sharply in 2021, UK households’ spending powers have plummeted. It would be reasonable to assume that these increasingly hostile economic conditions have caused fuel poverty rates to rise.
However, according to the Lilee fuel poverty metric, in England there have only been modest changes in fuel poverty incidence year on year. In fact, government statistics show a slight decrease in the nationwide rate, from 13.2% in 2020 to 13.0% in 2023.
Our recent study suggests that these figures are incorrect. We estimate the rate of fuel poverty in England to be around 2.5 times higher than what the government’s statistics show, because the criteria underpinning the Lilee estimation process leaves out a large number of financially vulnerable households which, in reality, are unable to afford and maintain adequate warmth.

Energy security
In 2022, we undertook an in-depth analysis of Lilee fuel poverty in Greater London. First, we combined fuel poverty, housing and employment data to provide an estimate of vulnerable homes which are omitted from Lilee statistics.
We also surveyed 2,886 residents of Greater London about their experiences of fuel poverty during the winter of 2022. We wanted to gauge energy security, which refers to a type of self-reported fuel poverty. Both parts of the study aimed to demonstrate the potential flaws of the Lilee definition.
Introduced in 2019, the Lilee metric considers a household to be “fuel poor” if it meets two criteria. First, after accounting for energy expenses, its income must fall below the poverty line (which is 60% of median income).
Second, the property must have an energy performance certificate (EPC) rating of D–G (the lowest four ratings). The government’s apparent logic for the Lilee metric is to quicken the net-zero transition of the housing sector.
In Sustainable Warmth, the policy paper that defined the Lilee approach, the government says that EPC A–C-rated homes “will not significantly benefit from energy-efficiency measures”. Hence, the focus on fuel poverty in D–G-rated properties.
Generally speaking, EPC A–C-rated homes (those with the highest three ratings) are considered energy efficient, while D–G-rated homes are deemed inefficient. The problem with how Lilee fuel poverty is measured is that the process assumes that EPC A–C-rated homes are too “energy efficient” to be considered fuel poor: the main focus of the fuel poverty assessment is a characteristic of the property, not the occupant’s financial situation.
In other words, by this metric, anyone living in an energy-efficient home cannot be considered to be in fuel poverty, no matter their financial situation. There is an obvious flaw here.
Around 40% of homes in England have an EPC rating of A–C. According to the Lilee definition, none of these homes can or ever will be classed as fuel poor. Even though energy prices are going through the roof, a single-parent household with dependent children whose only income is universal credit (or some other form of benefits) will still not be considered to be living in fuel poverty if their home is rated A-C.
The lack of protection afforded to these households against an extremely volatile energy market is highly concerning.
In our study, we estimate that 4.4% of London’s homes are rated A-C and also financially vulnerable. That is around 171,091 households, which are currently omitted by the Lilee metric but remain highly likely to be unable to afford adequate energy.
In most other European nations, what is known as the 10% indicator is used to gauge fuel poverty. This metric, which was also used in England from the 1990s until the mid 2010s, considers a home to be fuel poor if more than 10% of income is spent on energy. Here, the main focus of the fuel poverty assessment is the occupant’s financial situation, not the property.
Were such alternative fuel poverty metrics to be employed, a significant portion of those 171,091 households in London would almost certainly qualify as fuel poor.
This is confirmed by the findings of our survey. Our data shows that 28.2% of the 2,886 people who responded were “energy insecure”. This includes being unable to afford energy, making involuntary spending trade-offs between food and energy, and falling behind on energy payments.
Worryingly, we found that the rate of energy insecurity in the survey sample is around 2.5 times higher than the official rate of fuel poverty in London (11.5%), as assessed according to the Lilee metric.
It is likely that this figure can be extrapolated for the rest of England. If anything, energy insecurity may be even higher in other regions, given that Londoners tend to have higher-than-average household income.
The UK government is wrongly omitting hundreds of thousands of English households from fuel poverty statistics. Without a more accurate measure, vulnerable households will continue to be overlooked and not get the assistance they desperately need to stay warm.
Torran Semple receives funding from Engineering and Physical Sciences Research Council (EPSRC) grant EP/S023305/1.
John Harvey does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
european uk pandemicInternational
Looking Back At COVID’s Authoritarian Regimes
After having moved from Canada to the United States, partly to be wealthier and partly to be freer (those two are connected, by the way), I was shocked,…

After having moved from Canada to the United States, partly to be wealthier and partly to be freer (those two are connected, by the way), I was shocked, in March 2020, when President Trump and most US governors imposed heavy restrictions on people’s freedom. The purpose, said Trump and his COVID-19 advisers, was to “flatten the curve”: shut down people’s mobility for two weeks so that hospitals could catch up with the expected demand from COVID patients. In her book Silent Invasion, Dr. Deborah Birx, the coordinator of the White House Coronavirus Task Force, admitted that she was scrambling during those two weeks to come up with a reason to extend the lockdowns for much longer. As she put it, “I didn’t have the numbers in front of me yet to make the case for extending it longer, but I had two weeks to get them.” In short, she chose the goal and then tried to find the data to justify the goal. This, by the way, was from someone who, along with her task force colleague Dr. Anthony Fauci, kept talking about the importance of the scientific method. By the end of April 2020, the term “flatten the curve” had all but disappeared from public discussion.
Now that we are four years past that awful time, it makes sense to look back and see whether those heavy restrictions on the lives of people of all ages made sense. I’ll save you the suspense. They didn’t. The damage to the economy was huge. Remember that “the economy” is not a term used to describe a big machine; it’s a shorthand for the trillions of interactions among hundreds of millions of people. The lockdowns and the subsequent federal spending ballooned the budget deficit and consequent federal debt. The effect on children’s learning, not just in school but outside of school, was huge. These effects will be with us for a long time. It’s not as if there wasn’t another way to go. The people who came up with the idea of lockdowns did so on the basis of abstract models that had not been tested. They ignored a model of human behavior, which I’ll call Hayekian, that is tested every day.
These are the opening two paragraphs of my latest Defining Ideas article, “Looking Back at COVID’s Authoritarian Regimes,” Defining Ideas, March 14, 2024.
Another excerpt:
That wasn’t the only uncertainty. My daughter Karen lived in San Francisco and made her living teaching Pilates. San Francisco mayor London Breed shut down all the gyms, and so there went my daughter’s business. (The good news was that she quickly got online and shifted many of her clients to virtual Pilates. But that’s another story.) We tried to see her every six weeks or so, whether that meant our driving up to San Fran or her driving down to Monterey. But were we allowed to drive to see her? In that first month and a half, we simply didn’t know.
Read the whole thing, which is longer than usual.
(0 COMMENTS) budget deficit coronavirus covid-19 white house fauci trump canadaInternational
Problems After COVID-19 Vaccination More Prevalent Among Naturally Immune: Study
Problems After COVID-19 Vaccination More Prevalent Among Naturally Immune: Study
Authored by Zachary Stieber via The Epoch Times (emphasis…
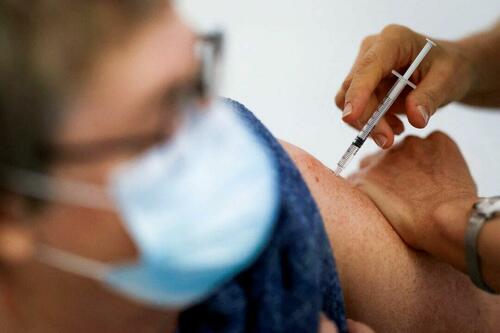
Authored by Zachary Stieber via The Epoch Times (emphasis ours),
People who recovered from COVID-19 and received a COVID-19 shot were more likely to suffer adverse reactions, researchers in Europe are reporting.
Participants in the study were more likely to experience an adverse reaction after vaccination regardless of the type of shot, with one exception, the researchers found.
Across all vaccine brands, people with prior COVID-19 were 2.6 times as likely after dose one to suffer an adverse reaction, according to the new study. Such people are commonly known as having a type of protection known as natural immunity after recovery.
People with previous COVID-19 were also 1.25 times as likely after dose 2 to experience an adverse reaction.
The findings held true across all vaccine types following dose one.
Of the female participants who received the Pfizer-BioNTech vaccine, for instance, 82 percent who had COVID-19 previously experienced an adverse reaction after their first dose, compared to 59 percent of females who did not have prior COVID-19.
The only exception to the trend was among males who received a second AstraZeneca dose. The percentage of males who suffered an adverse reaction was higher, 33 percent to 24 percent, among those without a COVID-19 history.
“Participants who had a prior SARS-CoV-2 infection (confirmed with a positive test) experienced at least one adverse reaction more often after the 1st dose compared to participants who did not have prior COVID-19. This pattern was observed in both men and women and across vaccine brands,” Florence van Hunsel, an epidemiologist with the Netherlands Pharmacovigilance Centre Lareb, and her co-authors wrote.
There were only slightly higher odds of the naturally immune suffering an adverse reaction following receipt of a Pfizer or Moderna booster, the researchers also found.
The researchers performed what’s known as a cohort event monitoring study, following 29,387 participants as they received at least one dose of a COVID-19 vaccine. The participants live in a European country such as Belgium, France, or Slovakia.
Overall, three-quarters of the participants reported at least one adverse reaction, although some were minor such as injection site pain.
Adverse reactions described as serious were reported by 0.24 percent of people who received a first or second dose and 0.26 percent for people who received a booster. Different examples of serious reactions were not listed in the study.
Participants were only specifically asked to record a range of minor adverse reactions (ADRs). They could provide details of other reactions in free text form.
“The unsolicited events were manually assessed and coded, and the seriousness was classified based on international criteria,” researchers said.
The free text answers were not provided by researchers in the paper.
“The authors note, ‘In this manuscript, the focus was not on serious ADRs and adverse events of special interest.’” Yet, in their highlights section they state, “The percentage of serious ADRs in the study is low for 1st and 2nd vaccination and booster.”
Dr. Joel Wallskog, co-chair of the group React19, which advocates for people who were injured by vaccines, told The Epoch Times: “It is intellectually dishonest to set out to study minor adverse events after COVID-19 vaccination then make conclusions about the frequency of serious adverse events. They also fail to provide the free text data.” He added that the paper showed “yet another study that is in my opinion, deficient by design.”
Ms. Hunsel did not respond to a request for comment.
She and other researchers listed limitations in the paper, including how they did not provide data broken down by country.
The paper was published by the journal Vaccine on March 6.
The study was funded by the European Medicines Agency and the Dutch government.
No authors declared conflicts of interest.
Some previous papers have also found that people with prior COVID-19 infection had more adverse events following COVID-19 vaccination, including a 2021 paper from French researchers. A U.S. study identified prior COVID-19 as a predictor of the severity of side effects.
Some other studies have determined COVID-19 vaccines confer little or no benefit to people with a history of infection, including those who had received a primary series.
The U.S. Centers for Disease Control and Prevention still recommends people who recovered from COVID-19 receive a COVID-19 vaccine, although a number of other health authorities have stopped recommending the shot for people who have prior COVID-19.
Another New Study
In another new paper, South Korean researchers outlined how they found people were more likely to report certain adverse reactions after COVID-19 vaccination than after receipt of another vaccine.
The reporting of myocarditis, a form of heart inflammation, or pericarditis, a related condition, was nearly 20 times as high among children as the reporting odds following receipt of all other vaccines, the researchers found.
The reporting odds were also much higher for multisystem inflammatory syndrome or Kawasaki disease among adolescent COVID-19 recipients.
Researchers analyzed reports made to VigiBase, which is run by the World Health Organization.
“Based on our results, close monitoring for these rare but serious inflammatory reactions after COVID-19 vaccination among adolescents until definitive causal relationship can be established,” the researchers wrote.
The study was published by the Journal of Korean Medical Science in its March edition.
Limitations include VigiBase receiving reports of problems, with some reports going unconfirmed.
Funding came from the South Korean government. One author reported receiving grants from pharmaceutical companies, including Pfizer.
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoAll Of The Elements Are In Place For An Economic Crisis Of Staggering Proportions
-

 International1 week ago
International1 week agoEyePoint poaches medical chief from Apellis; Sandoz CFO, longtime BioNTech exec to retire
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoCalifornia Counties Could Be Forced To Pay $300 Million To Cover COVID-Era Program
-

 Uncategorized3 weeks ago
Uncategorized3 weeks agoApparel Retailer Express Moving Toward Bankruptcy
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoIndustrial Production Decreased 0.1% in January
-

 International7 days ago
International7 days agoWalmart launches clever answer to Target’s new membership program
-

 Spread & Containment2 days ago
Spread & Containment2 days agoIFM’s Hat Trick and Reflections On Option-To-Buy M&A
-

 Uncategorized4 weeks ago
Uncategorized4 weeks agoRFK Jr: The Wuhan Cover-Up & The Rise Of The Biowarfare-Industrial Complex





















